2016 IMS Asia-Pacific Insight Magazine
- 1. Inside this issue • Understanding opportunity in Myanmar’s healthcare market • The next battleground for consumer health companies in Asia Pacific • The expanding value of observational studies • Navigating China’s growing medical device market IMS Asia-Pacific InsightIssue 6 | 2016
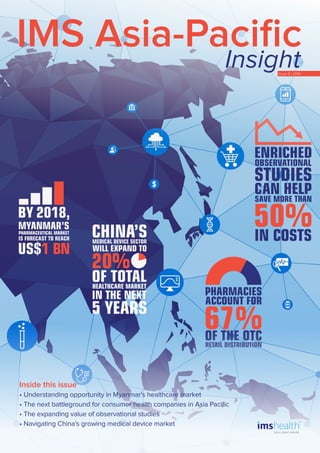
- 2. 2 Myanmar in transition Understanding opportunity in Myanmar’s healthcare market...............................................................................................5 After decades in isolation, Myanmar’s emergence onto the world stage is generating tremendous excitement, especially for healthcare players who are eager to bring better healthcare outcomes to a population with significant need. However, there are significant challenges that cannot be underestimated. This article reviews the infrastructure, talent, and regulatory hurdles that will challenge healthcare multinationals to re-evaluate what it means to enter this Frontier Market. Channel management The next battleground for consumer health companies in Asia Pacific......................................................................... 11 The consumer health market is evolving rapidly, nowhere more so than in the retail environment. This article examines the retail trends exerting the most pressure in Asia Pacific, and how those trends are reshaping channel dynamics and strategies for companies. Building a more complete picture The expanding value of observational studies........................................................................................................................... 17 Increasingly, healthcare stakeholders are demanding a more complete picture of the economic, clinical and personal value of drugs and medical services. Here, we discuss the expanding potential of Enriched Observational Studies to complement clinical evaluations, both in Asia Pacific and around the world. Policies, pressure and potential Navigating China’s growing medical device market...........................................................................................................21 The medical device market in China is evolving into a robust growth sector. However, a range of new policies are poised to exert significant pressure on domestic and MNC firms alike. This article outlines this ‘new normal’ environment, and how companies can — and should — adjust to capture opportunity. Contents
- 3. Welcome to the 6th edition of IMS Asia-Pacific Insight. As pharmaceutical markets follow a general trend of economic slowdown, both globally and here in Asia Pacific, finding innovative and creative pockets of opportunity has never been more critical. Therefore, this issue of Asia Pacific Insight magazine focuses on the theme of “what’s next?” New areas of play, new methodologies and even wholly new geographies are poised to disrupt the healthcare landscape in this region and fundamentally change how and where businesses operate. Furthermore, how stakeholders respond to this sea- change — including their appetite for changes in their own role and skill set — will be crucial to their success in the coming years. For some, this may mean re-defining what success means to your organization. In Myanmar, for example, our leading-edge analysis of this exciting, but tenuous market urges decision makers to critically manage operational expectations around reality, rather than aspirations. For others, such as those in consumer health, a rapidly evolving landscape is dramatically changing the rules of engagement, demanding fast adoption of new strategies, skills and even business models. And finally, articles discussing an enhanced approach to generating robust RWE and a “new normal” environment for medical device companies in China demonstrate the value of information in generating better outcomes and strategies. Asia Pacific continues to be a tremendously diverse healthcare environment and as such demands informed expertise and insights to navigate. IMS Health is committed to providing our clients in this region with the information, analysis, and intelligent perspective necessary to identify opportunity and cultivate success. Welcome letter
- 4. 4
- 5. 5 Myanmar in transition Understanding opportunity in Myanmar’s healthcare market Amid a general slowdown of the global pharmaceutical market, pharmerging markets continue to be a formidable engine of growth; across Asia, Africa and South America, these markets are boasting a CAGR of 10-14%. At the outer edges of these growing economies are markets identified by IMS Health as “Frontier Markets” – the next big drivers of growth, opportunity and even innovation. Among the Frontier Markets, Myanmar is capturing the most attention. With the completion of parliamentary elections in early November 2015, there are positive signs that the momentum for change and market liberalization will accelerate. However, Myanmar’s underinvested healthcare infrastructure, sizeable talent gaps, and significant regulatory and affordability hurdles, require an informed approach, and managed expectations. Multinationals will be challenged to re-evaluate what it takes to play, and what it means to win, in both the short and long term. Myanmar: A positive outlook As Myanmar embraces a market economy, the country is gradually gaining ground. GDP and life expectancy have both been growing steadily, with salaries, disposable income and economic opportunities all on the rise. For foreign investors, signs of economic and political reform are generating confidence in continued growth and market expansion. Indeed, there appears to be only one direction to go – upward. However, this growth will certainly be organic, and prolonged. “Myanmar has embarked on a relatively swift and pronounced politico-economic change of direction. The examples of countries that have followed similar paths of transformation over the last 30 years should inform a sense of optimism as well as caution,” says Tim Walton, Senior Principal, Asia Emerging Markets, IMS Health. A young, but promising, healthcare market As the political and social environment has stabilized over the past five years, government spending on healthcare has concurrently increased (Fig 1). A comparison to other markets in Southeast Asia, though, where spending as a percentage of GDP is typically at 3-4%, demonstrates that the current baseline is still extremely low 1 . Nevertheless, increases in government expenditure, together with direct investment from regional players and pioneering MNCs has led to the significant growth of the healthcare market in recent years; the pharmaceutical market alone is forecast to reach US$1 billion by 2018. And so an alluring picture of untapped potential emerges for multinational companies (MNCs), who are captivated by the opportunity to take an early-mover advantage, establish a presence in a significant Frontier Market, and play a role in bringing better health outcomes to a population with significant need. However, this picture 1 WHO, World Health Statistics 2014 Fig.1 Healthcare spending in Myanmar 2010-2015 Healthcare spending as a % of GDP Healthcare spending as a % of overall govt expenditure 4 3.5 3 2.5 2 1.5 1 0.5 0 2010-2011 Percent 2012-20132011-2012 2013-2014 2014-2015 1.05 0.2 0.21 0.76 0.89 0.991.05 2.82 3.15 3.38 Source: Ministry of Health, Health in Myanmar 2014, www.moh.gov.mm
- 6. 6 must be set in the context of appropriately-managed expectations. Companies wanting to take a position in Myanmar today must do so with a long-term strategic vision and an understanding that a significant ROI can only be the result of sustained investment over an extended planning cycle. Preparing for an uphill climb Enthusiasm, therefore, needs to be tempered by an informed understanding of the market’s significant challenges. Through its operations in Myanmar, and extensive discussions with clients and partners in the region, IMS Health has identified three critical barriers for MNCs: Infrastructure Myanmar’s healthcare infrastructure is spread over a broad network of hospitals, health centers and other points of care with varying degrees of capability. Within these physical structures, there are also significant gaps in quality and functionality. Understanding the hierarchy of access points, and the conditions therein, is a necessary first step in developing a holistic view of the healthcare landscape. Points of care Healthcare delivery in Myanmar ranges from sub-rural health centers (RHCs), largely focused on ambulatory and primary care, to urban tertiary centers of expertise (Fig.2). For a population of just over 51 million people, there are a total of 827 primary care hospitals, 81 secondary care hospitals and 36 tertiary centers.2 And while Myanmar’s ratio of hospital beds per 1,000 people (.06 in 2010) is low, there have been significant improvements over the past decade; a 17.4% increase in public hospitals and a 12.6% increase in RHCs from 2004 to 2013.3 However, the vast majority of the country’s rural poor population is without access to these facilities, either due to cost or geography. Furthermore, many hospitals, especially at the primary/rural level, are without necessary ancillary services or technologies, such as lab facilities or diagnostic imaging. Conditions Compounding the shortage of facilities are substandard conditions. Buildings — even private clinics — often lack basic functions, such as constant electricity and water supply, which can result in unhygienic environments. Drug storage is in a similarly rudimentary state, with limited cold chain refrigeration, frequent power outages and open, disorganized shelves. The wholesale markets, it should be noted, do not offer significant improvements, and employ stock control/management systems that are chaotic at best. Talent pool Aside from the infrastructure gaps, sufficient human resources for healthcare is cited as the greatest challenge, and thus highest priority, for incoming pharmaceutical companies. The lack of a qualified talent pool can be found at both the corporate/commercial level, as well as at the healthcare provider (HCP) level. “The talent gap is the inevitable consequence of a society that has lived under isolation and sanctions for so many years,” explains Mr. Walton. “Having a people strategy that transfers knowledge and develops talent will be key to long term growth.” 2 National census, 2014; Health systems. 3 Health Systems, p 80 An insufficient talent pool (on both the corporate and provider level) that is inexperienced at best, nonexistent at worst High affordability hurdles An under-funded and over-burdened healthcare infrastructure Note: This figure does not include military hospitals. In 2007, there were 29 military hospitals and 14 field medical battalions across Myanmar. Tertiary level Urban hospital (Yangon, Mandalay, Nay Pyi Taw · 1000-bed · 20 disciplines · Intensive care Rural hospital Sub-rural health center (Sub-RHC) · Basic health staff · Ambulatory · Emergency · 16-bed · Emergency · General primary care Township hospital District hospital · Primary/general care · Specialty disciplines: anesthesia, orthopedic, eye, dental, pathology · 25-bed · Aux/admin services · General medicine, surgery, obstetrics, gynaecology, pediatric Secondary level Primary level Fig.2 Myanmar's healthcare Infrastructure
- 7. 7 Corporate/commercial talent gaps Upon entering Myanmar, multinational companies will find that employees in the healthcare sector have thus far operated with little to no regulations or systematic guidelines, and have no experience with the sophisticated HR, finance or even (notably) pharmacovigilance functions of a multinational company. And while local talent has demonstrated to be both willing and able to embrace a more structured corporate model, the lack of formal training and the need for guidance will be a hurdle to generating short-term momentum. Healthcare practitioner talent gaps Of around 20,000 licensed medical doctors nationwide, IMS Health’s latest estimate is that the prescription market is driven by 4,000 qualified and practicing physicians across 7 urban areas. The significant majority (80%) is concentrated in Yangon and Mandalay (Fig 3). And while the overall number of HCPs (including nurses and midwives) has been steadily increasing since the early 2000s, it has not yet reached the global standard of 2.28 per 1,000 population 4 . Initial training for medical professionals is standardized by the Ministry of Health’s Department of Medical Science, with a number of medical universities and post-graduate programs offering degrees in a range of specialties. However, in practice, providers are primarily focused on internal medicine, with little-to-no continuing education or hands-on specialty experience. This lack of specialty expertise places an enormous burden on internal medicine physicians, who often treat patients above and beyond their capability set. Lack of medical knowledge in Myanmar is a function of both distance and necessity. Doctors have been, until recently, almost completely disconnected from the global medical community; internet access is subpar, and travel outside Myanmar had been restricted. This has resulted in a pervasive lack of awareness about new products, treatment protocols and disease pathways. But the root cause is largely economic – for a doctor to earn sufficient money, volume and consultations must take priority over new products or specialty patients. Affordability With only 4% of Myanmar’s population considered part of the “consuming class,” affordability is a significant bottleneck to market growth5 . Today, the majority of Myanmar citizens are poor, with extremely limited access – geographic and financial – to even the most basic healthcare services. Geographically, as discussed, the services closest to most people are confined to basic or emergency care. For specialty treatment, expertise and facilities are both prohibitively far away. Financially, as the bulk of expenses must be paid out of pocket, cost tends to drive decisions about where, and how, to be treated (if at all). On the other side of the affordability spectrum, Myanmar is losing significant patient numbers from the top of the pyramid to nearby countries such as Thailand, India and Singapore, especially for complex and expensive treatments such as oncology or transplants. Such out-bound ‘medical tourism’ poses an obvious challenge (and opportunity) for Myanmar’s domestic healthcare industry and pharma companies, who must work hard to retain this “willing-to-pay” population, and to ensure adequate provision for after-care. 4 Health Systems, p 78 5 McKinsey Global Institute. “Myanmar’s Moment: Unique opportunities, major challenges.” June 2013 Mandalay 24% Nay Pyi Taw 8% Lashio 2% Taunggyi 2% Mawlamyine 4% Yangon 56% Magwe 4% Fig. 3 Practicing physicians by urban area Source: IMS Health primary research 2015
- 8. 8 Playing to win Ultimately, MNCs will need to develop educated strategies that embrace the reality of what success might look like for their bottom line – there is currently a small pie to share. And while an underlying challenge in such a unique market is that “best practice” doesn’t always apply, certain strategies will be core to any successful, and sustainable, approach: 1. Build a tailored portfolio Already, Myanmar is becoming a crowded marketplace in some areas; opportunity is there, but must be captured swiftly and strategically. To succeed, incoming pharma MNCs will need to thoughtfully choose a road: - Primary care portfolios. Low cost products (e.g. antibiotics) carry high volume potential. These strategies will require less investment in infrastructure, but perhaps more advanced brand planning to understand unmet need and pockets of opportunity. √ Build a larger basket of brands to maximize efficiencies in promotional spend √ Develop a qualified sales team and a willingness to expand beyond urban areas - Specialty portfolios. Small but profitable niche markets (e.g. oncology, hepatitis) may offer high returns, but also low volumes. Such an approach will require a large enough affordable patient population to justify the investment. √ Focus on differentiated therapy areas that can transform or establish a targeted market √ Invest in education programs and pre-launch patient population research 2. Invest in people Clearly a high area of need, and opportunity, is Myanmar’s human capital. As discussed, this need falls into two categories: corporate talent and healthcare providers. For corporate talent: √ Focus on training and development √ Ensure recruitment, training and ongoing development is done on the ground, not remotely, to foster a mutual understanding of culture, expectations and desired outcomes For healthcare providers: √ Develop focused, continual training (most of which will be a new experience) √ Training should take place in a variety of settings, including at the point of care There is, however, a third area of opportunity that should be considered in support of a long term commitment — patient education and empowerment. A better educated, more informed provider population will be a critical catalyst for driving uptake and sustainable adoption of medicines, especially for newer or lesser- known disease areas. Patients represent a powerful opportunity for pharma companies to round out their footprint in Myanmar. To date, very little has been done to support or educate this population, as there is often an ethical hesitation to communicate directly with patients. However, through healthcare professionals, associations and other community networks, pharma companies can use patient education as a complement to more traditional people investments. 3. Cultivate a network Particularly in areas that require extensive education and awareness campaigns, the ability and willingness to connect and collaborate with local stakeholders and known key opinion leaders (KOLs) is extremely valuable. This is especially true among the provider community. In Myanmar, which is highly hierarchical, doctors are placed at the top, alongside religious and military figures. As such, they occupy significant positions of authority and influence within both professional and community circles. Tapping into this structure through an informed KOL strategy, therefore, will be extremely important in establishing necessary brand equity to drive longer-term ROI. Myanmar presents a plethora of exciting opportunities for the global pharma community; such an untapped market is both rare and short-lived. Positive growth projections, a stable political and economic base reinforced by recent democratic elections, and a growing consumer class are creating an almost overwhelming amount of enthusiasm. However, such enthusiasm should be eased by due diligence and perhaps reduced to a point of cautious, or at least realistic, optimism. Successful market entry in Myanmar will be built on an informed approach that thoughtfully addresses and prioritizes the country’s fundamental infrastructure, talent, and affordability challenges. To read our full white paper on this topic, which includes a deep dive analysis of Myanmar’s healthcare system, and the challenges facing MNCs as they approach this landscape, please contact: Tim Walton Senior Principal, Asia Emerging Markets, IMS Health twalton@sg.imshealth.com Ankit Kulshresta, Senior Consultant, Primary Intelligence at akulshresta@sg.imshealth.com Summary
- 9. Make the most of every conversation Marketing Social MobiIe Intelligence Performance Incentives Understanding your customers better, communicating with them across a variety of channels, having a common company view on the various interactions: this will help you to attract the right audience, engage in meaningful conversations on every occasion and refine your communications over time. The IMS Health Nexxus Commercial Application Suite is a set of interconnected applications for commercial functions in Life Sciences that will elevate your outreach. IMS HEALTH • www.imshealth.com 8 Cross Street #21-01/02/03/04, Singapore 048424 ©2016 IMS Health Incorporated and its affiliates. All rights reserved. Trademarks are registered in the United States and in various other countries. Life Sciences companies using Nexxus applications in combination with other IMS Health information and services are able to: • Reduce costs and operational complexity • Become more agile in responding to shifting market needs • Transform customer engagement into meaningful discussions • Derive actionable business insights from integrated data assets For more information, contact our team at +65 6227 3006 or apac.info@imshealth.com
- 10. 10
- 11. 11 Channel management The next battleground for consumer health companies in Asia Pacific The consumer health market is proving to be an extraordinary pocket of opportunity in Asia Pacific. This is not necessarily surprising. Driven by a myriad of social and cultural changes, consumers are increasingly invested in managing their own health - and doing so outside traditional points of care. What is surprising is the pace at which the market is evolving, and how that change is disrupting business models across the value (and supply) chain. Nowhere is this more apparent than in the retail environment, which is fast becoming a priority battleground for consumer health companies. Trends such as pharmacy consolidation, the rise of convenience stores and modern trade, and the emergence of innovative new channels (e.g. e-commerce) are converging to inspire a ‘re-think’ of everything from product distribution and consumer engagement, to the internal capabilities necessary to execute successful strategies. This article examines the retail trends exerting the most pressure in Asia Pacific, and discusses how those trends are reshaping channel dynamics for consumer health players. Around the world, consumer health manufacturers and marketers are paying more and more attention to retail environments – and with good reason. Some of the most disruptive trends in healthcare are fundamentally shifting not only where consumers buy medicine and wellness products, but also why and how they make that choice. Proactive, informed channel management, then, has become an immensely important area of play. In Asia, the challenge is, as usual, complex. Here, retail channels are extremely fragmented and there is a wide variety of models in play. “This is already an incredibly complex environment that has seen a number of critical changes in recent years,” explains Anthony Morton-Small, Senior Principal, IMS Consulting Group. “Now, the shifts in channel structure and the emergence of disruptive new channels – much of which we’ve already seen in Western markets of Europe and North America – will really challenge companies to evolve their strategies and dramatically elevate their capabilities.” IMS Health has identified three core trends that are poised to have the greatest impact on channel management for consumer health companies in Asia Pacific: 1. Pharmacy consolidation 2. The rise of convenience stores and modern trade 3. The emergence of new channels Together, these forces will transform what it means to play, and ultimately what it will take to win, in the region’s retail channels. Pharmacy consolidation It is a familiar story in retail: changes in shopping behavior, the pressures of modernization, and declining trade margins force (either organically or disruptively) the decline of independent shops and small businesses, and give rise to consolidated chains and big businesses. In the pharmacy channel, however, this process has unique implications; changes here are both a signal and a driver of fundamental shifts in how consumers – and by extension companies – manage and engage with healthcare delivery. In Asia, we’ve seen three distinct developments that are shaping a more consolidated pharmacy landscape. Understanding the dynamics of these levers, and their impact on business models, is essential for consumer health companies as they seek to optimize opportunity in this traditional channel.
- 12. 12 1. The consolidation of independent pharmacies One of the key characteristics of an independent pharmacy is a reliance on service over product. In this environment, the pharmacist is at the center; offering advice and marketing personal reputation and community standing – which are the primary forces of differentiation. Such a model yields high loyalty, but low footfall and sales per store. Volume, or rather the lack thereof, is also an issue; this model suffers from eroding high margins and decreased buying power with wholesalers. It is not surprising, then, that as larger chains have emerged in urban areas, the number of independent pharmacies has declined. To remain relevant and competitive, individual pharmacies are opting to form cooperative buying groups to negotiate better margins from wholesalers, while still maintaining unique product selection and service levels in their stores. Significantly, how these affiliations manage their consumer-facing brand differs across markets. In Australia, banner groups1 such as Guardian, AMCAL and Terry White Chemists have a similar look-and-feel across all stores. In South Korea, on the other hand, individual pharmacy owners still control the appearance of their own stores. 2. The rise of pharmacy chains The chain model enables a more uniform, and ultimately a more profitable, approach for many pharmacies. Strategic cost management and higher buying power with manufacturers ensures competitive pricing and, subsequently, higher sales per store. The management of such a model, however, is, and must be, fundamentally different from the ‘mom and pop’ shop; while pharmacist services are still important, direct marketing of OTC products is also necessary. Additionally, strong store branding (disconnected from the reputation of an individual pharmacist), membership discounts and longer hours make chain pharmacies very different physical environments for both companies and consumers. 3. The professionalization of retailing, merchandising & buying To date, many companies have been able to operate on a somewhat informal basis, using experience and relationships to dictate ‘one-off’ strategies with independent pharmacies. However, with the evolution of the channel and the growth of pharmacy chains, a more sophisticated, more corporate approach is required. To succeed, and capture a meaningful share of voice – and share of shelf – in larger chain businesses, companies must embrace the subtle shifts in success levers across the marketing 4Ps: product, place, promotion, and price. “Companies need to understand that to play in this new arena, new skills are going to be required. They need to evolve beyond just selling to stores and towards selling through stores, and take a much more strategic and disciplined approach to key accounting management, retail engagement and point-of-sale activation,” says Mr. Morton-Small. Convenience stores & modern trade The influx of consumers into cities over the past decade, and the subsequent growth of a busier, more demanding consumer culture, has transformed the retail landscape. Convenience stores, in particular, have become incredibly prevalent, surpassing pharmacies and drugstores as the primary point of sale not only for daily necessities, but also for consumer health products. South Korea, for example, has seen the successful entry of convenience stores into the OTC market, and in Thailand the mighty 7-11 convenience chain is currently experimenting with both standalone pharmacies and pharmacy shop-in-shops. Also on the rise (though at a slower pace of growth) are large, multi-site corporate retailers. These ‘modern trade’ players, such as Giant and Carrefour, represent an entirely new model serving the urban Asian consumer. A new consumer There has also been a qualitative shift in how consumers are engaging with their own health, largely due to increased media investment and growing government policy reforms regarding health management. Media campaigns, including advertisements, magazines, and blogs, have been particularly powerful in raising awareness about the impact of unhealthy lifestyles. As a result, consumers are increasingly interested in, and capable of, maintaining their own health. They are also demanding a wider range of settings in which to purchase health products, integrating ‘health shopping’ with general purchases such as food and even cosmetics. New channels While the pharmacy, convenience and modern trade channels are challenging consumer health players to adjust, wholly new channels such as mobile and internet retailing are demanding a 360-degree transformation in how, and where, business is conducted. “E-channels and direct selling models are growing rapidly in this part of the world. In China, for example, both e-tailing and direct selling of OTC medications and nutritional products are seeing 100% year on year growth rates,” confirms Veronita Rusli, Senior Manager, Consumer Health, IMS Health. “It would be a costly mistake to assume that these emerging markets do not already have a firm grasp on innovative digital models.” Indeed, many companies in the region are already 1 “Banner group”: A cooperative association of individually-owned pharmacies who join together to negotiate better prices with wholesalers, and to share support for marketing, advertising and promotional services.
- 13. 13 exploring entirely new brand and supply chain strategies driven by online platforms. Alibaba’s Alipay™ is a prime example, offering payment, logistics and marketing solutions that enable unprecedented access to, and for, the Chinese market. This model has completely revolutionized how demand can be satisfied across the value chain. In Asia, as in the rest of the world, the emergence of these new channels has come ahead of regulation and policy frameworks. Such a dynamic has allowed business models to develop quickly, but it has also meant that there are significant unresolved issues on the horizon that could threaten long term sustainability, including: • Taxation of internet sales • Cross-border shipping regulations • Lack of prescription drug control in e-tailing environments However, this lack of policy infrastructure is also a profound opportunity. Consumer health companies in Asia have the unique chance to help shape future policy, both to their advantage and to the advantage of burgeoning markets. Action Steps The evolution of retail channel dynamics in Asia Pacific discussed here will ultimately force a sea change in how and where consumer health products are distributed. To proactively and sustainably grow in this new landscape, companies will need to critically consider the following action steps. 1. Anticipate and evaluate channel development regionally, but tailor implementation at the local/country level (Fig. 1) While the overall trend in the region is moving toward a consolidation of pharmacy and other retail channels, each country is at a different stage. Implementation strategies should embrace both a macroscopic understanding of the models in play and specific activities tailored to specific markets. 2. Understand the changing role of stakeholders, and the implications for commercial success (Fig. 2) In this evolving channel, stakeholder roles across the value chain are shifting: Consumers are becoming more self empowered and less reliant on pharmacists' advice; pharmacists are acting more as business partners than product pushers; and marketers are increasingly Fig. 1 Otc distribution channel mix (country average across Asia Pacific) Manufacturers Sales outlets/Channels Hospitals & Clinics (〜2%) OTC drugs, including VMS Pharmacies / Drugstores (48%) All categories Modern trades2 (〜6%) All categories Traditional grocer (3%) Mostly "functional foods" Other non- store retailing3 (〜13%) All categories VMS Direct selling (28%) Distributors & Wholesalers DKSH, Zuellig, Mega, Tedis, Saphaco Product Price Place Promotion Product selling Product pricing Investment Product SKU Margin Marketing spend Buying point From To Selling product into a single store Selling products B2B and B2C Fixed pricing Variable pricing Fixed investment Flexible resource allocation Full range of products Fast selling SKUs High margins (individual purchaser) Low margins (group purchasing) High field force spend Trade marketing spend Multiple small buying points Single large buying point Fig. 2 Moving from product-centric to consumer-centric strategies 2 Modern trades include convenience stores, supermarkets, hypermarkets; 3 Non-store retailing includes direct selling, internet retailing, home shopping Source: Euro Monitor
- 14. 14 embracing a consumer-centric, rather than product- centric orientation. To maintain commercial success, companies will need to go back to basics, re-evaluating their approach along the four core principles of marketing; product, price, place and promotion. 3. Understand the key success factors to win in clinical, value and convenience channels In addition to addressing the change in stakeholder roles, commercial strategies will also need to accommodate the (increasing) variety of channel settings. Clinical, value and convenience model all carry different requirements for SKU ranges, pricing strategies and on-site marketing. Ultimately, the more tailored commercial activities can be to the environment, the greater the opportunity for market penetration and long term sustainability. 4. Understand the capabilities necessary in your organization to deliver more professionalized retailing, merchandizing and trade buying In many cases, this will require significant investment to either bring in new talent, or train existing resources. As we’ve seen in other regions, without this level of professionalization, any external commercial activities could face a potentially prohibitive uphill battle. 5. Evaluate the opportunity and disruptive potential of new online and direct selling channels The single-channel model is gradually becoming a symbol of the past. Looking ahead, successful strategies will engage not just multi-channel models, but also cross- channel and omni-channel strategies that engage customers and retailers on a higher, more sophisticated and more ubiquitous level. These new channels will converge in a holistic, 360-degree approach to consumer engagement and retailer management. Single channel • Customers experience a single type of touchpoint • Retailers have a single type of touchpoint • Customers experience a brand, not a channel within a brand • Retailers leverage their 'single view of the customer' in coordinated and strategic ways Multi-channel Cross-channel Omni-channel • Customers see multiple touchpoints acting independently • Retailers understand sales requirements for different channels, but activities are not coordinated • Customers see multiple touchpoints as part of the same brand • Retailers have a 'single view of the customer' but operate in functional silos Product recommendation x x On-site consultation x x Loyalty program x DTC campaign Merchandising x Low price x x High selling SKUs x x Key success factors (e.g Pharmacy shop-in-shop) Clinical (e.g. big box discount retailer) Value (e.g. 24/7 convenience stores) Convenience Driving account support and recommendations Managing accounts for growth Assessing appropriate stock weight Understanding account needs and expectations Maximizing shelf space and prime positions Building the relationship with the account Achieving listings and full ranging
- 15. 15 Anthony Morton-Small Senior Principal, IMS Consulting Group amorton-small@imscg.com Veronita Rusli Senior Manager, Consumer Health, IMS Health vrusli@sg.imshealth.com The consumer health retail landscape in Asia Pacific is undergoing a multi-faceted shift: Independent business models are increasingly moving toward consolidation; social and demographic shifts are empowering new players; and new technologies and channels are reshaping the very boundaries of the market. These changes will force consumer health companies to fundamentally change how, where and with whom they do business. Those companies who are not only able to adjust their strategies, but who are also willing to re-evaluate and re-orient their very business models, will be the ones that stay ahead of the pack. To read our full white paper on this topic, which includes a deep dive analysis of these trends, as well as key lessons from mature markets, please contact: Summary
- 17. 17 Building a more complete picture The expanding value of observational studies Increasingly, healthcare stakeholders are demanding a more complete picture of the economic, clinical and personal value of drugs and medical services. This trend has led to a proliferation of efforts on the part of regulators, payers and pharmaceutical companies to generate and capture Real-World Evidence (RWE) across the value chain. This article will examine the expanding potential of Enriched Observational Studies (EOS) to bring together two core elements of RWE: Economic insights and patient-reported outcomes. It will also discuss how EOS can - and should - complement clinical evaluation. Finally, it will review how this trajectory is playing out in the diverse markets of Asia Pacific, and where the challenges and opportunities in that landscape lie. A new value demand The demand to better measure and demonstrate value in healthcare reflects the convergence of several key trends. Together, these trends signal a critical shift from a narrow focus on treating disease, to a more holistic approach to health and wellness across the entire patient journey. 1. Regulators are requiring more long-term safety data gathered in real-world settings, taking into account co-morbidities and other lifestyle inputs. 2. Healthcare costs are under increasing pressure, both at the government level and at the corporate/R&D level, forcing more intense scrutiny on the cost and demonstrated value of medicines. 3. Payers are increasingly demanding outcomes- or value-based data to support reimbursement policies and decisions; randomized clinical trial outcomes are no longer sufficient in gauging the effectiveness of a treatment (read IMS Health’s 2015 article on Risk-Sharing Agreements in Asia Pacific for more insights on this trend). 4. The rise of patient-centric models is demanding a multi-faceted approach to treatment that addresses the full continuum of care, before, during and after treatment. This is particularly relevant for chronic illnesses; the aim has become to improve life, rather than just prolong it. As a result, Real-World Evidence (RWE) is garnering more and more attention, and is proving to be a critical component in both clinical and economic decisions about medicines. To generate this data, observational studies, which gather data from patients outside the clinical trial setting, and usually over a long period of time, are increasingly valuable. Observational studies: A more complete picture Observational studies (OS) have long been a standard methodology for understanding the long-term safety of medical treatments. The potential, however, is much broader. “We are witnessing a true paradigm shift in what data should be used and how it can be used,” explains Roy Leong, Head of Observational Studies, APAC, IMS Health. “Historically, observational studies generated data that was solely used for clinical evaluation. Now we are seeing the potential to use this methodology beyond that setting and gather valuable insights, especially around cost effectiveness and patient-reported outcomes.” An overview An observational study is one in which participants are not randomized or otherwise assigned to an exposure. The choice of treatments is up to the patients and their physicians. By nature, then, observational studies help to understand the impact of treatments in real-world, non-interventional settings, rather than clinical trials, thus bridging the gap between efficacy and effectiveness measurements. From a methodological standpoint, observational studies provide significant benefits over randomized clinical
- 18. 18 trials (RCTs), whose data is useful in assessing efficacy, but lacks “external generalizability” and is therefore generally inappropriate for measuring real-world effectiveness.1 This limitation is primarily the result of the patient population; RCTs are built around smaller, more restrictive groups that rarely represent the complexities of real-world users (Fig.1). Observational studies can include four types of studies that gather information across the life of a treatment (Fig.2): • Retrospective studies (a relatively new addition to the OS continuum) gather insights about patients prior to starting treatments, including prior diagnoses and treatments, lifestyle, etc. • Chart review studies collect medical data from past records • Cross-sectional studies collect information from an entire patient population at a specific point in time • Prospective studies collect baseline exposure information and then follow up with patients over time Ultimately, observational studies provide key insights into real-world applications that help build a much more complete picture of a drug’s impact. These insights can include: √ Patient compliance √ Physician prescribing behavior √ Co-morbidities √ Supplemental/additional drugs A new, expanded approach Conventional observational studies were limited to analyzing the long-term impact of treatments in large populations. This was a useful but narrow approach to understanding clinical safety. However, by expanding the focus of research to include economic insights and patient-reported outcomes, a definition of treatment value beyond the clinical setting is dramatically clarified. Furthermore, widening the time scope of the study, to include both mined retrospective data and prospective data in a single observational study, enables a truly longitudinal approach to value. This new approach is called Enriched Observational Studies (EOS). Cost-effectiveness In the face of rising cost pressures, regulatory, reimbursement and portfolio decisions are increasingly being linked to outcomes-based or value-based data. Data generated from EOS is extremely valuable in assessing, and therefore managing, the disease burden for all healthcare stakeholders. • For policy makers, EOS data can inform more streamlined resource allocation and spending both across therapy areas and within targeted patient populations. • For payers, real-world outcomes support a range of reimbursement decisions, increasing access for patients. This is especially true for innovative medicines where cost is high and effectiveness is, as yet, uncertain. • For manufacturers, EOS data builds a more accurate picture of unmet need, enabling focused, responsive R&D activities and generating higher returns on investment across the product lifecycle. It also critically informs price negotiations across markets. Enriched Observational Studies that bring together commercial and clinical perspectives can also provide critical evidence to support health economics and outcomes research (HEOR) such as cost of illness studies, economic modeling and other cost effectiveness analyses. 1 Payne, Krista., et al. Making the ‘MOST’ of Prospective Observational Studies. Evidence Matters Online; www.unitedbiosource.com; Fig. 2 Four types of studies applied in observational studies Fig. 1 Observational studies vs. conventional randomized clinical trials (RCTs) Observational studies Conventional RCT studies More flexible, they reflect real practice Strict inclusion criteria Patients might take more than one drug Restricted to only one treatment Co-morbidities Exclusion of patients within risk groups High sample size Small sample size Long follow up Short follow up Lower cost Expensive Retrospective studies Prospective studies Cross-sectional studies Chart review studies
- 19. 19 Patient-reported outcomes Patient-reported outcomes (PROs), gathered via interviews and validated questionnaires, and anonymized for privacy, have become a critical dimension in the generation of Real-World Evidence. Insights include valuable information about how treatments affect quality of life (for both the patient and caregiver), gained or lost productivity, ease of compliance, and other key dimensions of effectiveness. Roadblocks in Asia, and beyond Unfortunately, many health systems today lack the data, technology and regulatory framework to support the widespread adoption of Real-World Evidence and to use these new insights to affect real change. Asia Pacific, in particular, faces high barriers to understanding and embracing the value of RWE. Here, the multitude of systems, regulations and resources (or lack thereof) has hampered progress and left the region behind the rest of the world when it comes to generating and integrating new types of data into healthcare evaluations. How each country evaluates data, where that data comes from, and how much is available varies significantly across the region. The opportunity, however, is certainly there. IMS Health has identified three levers poised to act as powerful change agents in the region’s appetite and ability for an innovative approach to data and value-based evaluations of medicine: 1. Mature markets like Korea, Taiwan and Japan (and to a certain extent, China) are embracing new methodologies and robust Health Technology Assessments (HTAs). 2. International price referencing across many markets is elevating the demand for value-based data. 3. The rise of universal healthcare coverage goals in Vietnam and Indonesia is highlighting the need for more (and better) cost effectiveness studies in order to control healthcare spending. Similar themes can be found around the world. A recent roundtable between the Institute of Health Economics in Canada and AstraZeneca outlined a range of issues facing the future of Real-World Evidence, including • The lack of a clear policy framework for when and where RWE should be used • The disconnect between RWE, innovation and research • Limited participation in generating RWE across all stakeholder groups – particularly in aligning public and patient outcomes with policy priorities • Lack of common healthcare information technology (HIT) data structures and vocabularies These issues, while focused on Canada, are also relevant to other healthcare markets across both the developed and developing world, as they point to an overall lack of consistent, strategic investment in RWE as a standard in evaluating medicines.2 Recommendations While Enriched Observational Studies clearly represent a profound opportunity to elevate both the quality and quantity of value-based insights, they do not exist in a vacuum. To realize the maximum benefit from EOS, companies should consider the following: 1. Start early. Using EOS can build critical insights about patient populations, regulatory hurdles, and other potential challenges or opportunities that may affect pipeline or launch strategies. 2. Anticipate HTA obligations. Understanding what RWE will be necessary to support HTA submissions and other regulatory requirements enables a more directed and strategic investment in EOS. 3. Consider partnerships. Collaborating with established partners to host EOS (and subsequent disease registries) should be a key strategic consideration; partnerships can enable multiple stakeholders to meet both business and patient-related needs. 2 Nason, Eddy., Husereau, Don. Roundtable on Real World Evidence: System Readiness – Are we ready to use routinely collected data to improve health system performance. Summary Report, September 2014 A paradigm shift is underway in how the healthcare community understands, measures and demonstrates value. Traditional models of research, such as randomized clinical trials and standard market data reports, are increasingly giving way to Real-World Evidence, which delivers a more complete, and ultimately a more reliable, picture of the economic, clinical and personal value of a treatment. Enriched Observational Studies represent this new approach to value, combining commercial, clinical, and patient-reported data to build a better picture of outcomes. By integrating such studies, companies can more effectively negotiate, improve launch preparedness, and more accurately evaluate new markets and partnership opportunities. To continue the conversation, please contact: Roy Leong, Head of Observational Studies, APAC, IMS Health at chleong@sg.imshealth.com Summary
- 20. 20
- 21. 21 Policies, pressure and potential Navigating China's growing medical device market The medical device market in China has historically been a small, loosely regulated market that has lagged behind the larger pharma sector in terms of both maturity and accessibility. Today though, the tide is shifting; as the pharma market slows, the medical device market continues to grow and is expected to occupy an increasingly large portion of the total Chinese healthcare market. The future, however, will be a complex mix of opportunities and challenges. A range of new policies are poised to exert significant pressure on domestic and multinational firms alike, ultimately creating a “new normal” for the medical device industry. Understanding these policies, monitoring their progress, estimating their impact, and developing a coping strategy will be critical in navigating this market over the next five to ten years. Despite an overall economic slowdown, healthcare continues to be a significant area of focus and investment for China. The result has been a broadening of access and affordability, and the rise of a newly sophisticated patient population, both of which signal huge growth potential for the still under-developed medical device market: √ Increased government funding of insurance schemes, combined with rising disposable income and private insurance options, means improved affordability of medical devices. √ Increased investment in accessibility has grown the number of patients treated by a CAGR of 7% over the past 5 years. √ A growing interest and education in personal health has created demand for safer, higher quality medical devices to complement treatment regimens. As a result, IMS Consulting Group expects that the medical device sector will expand to account for 20% of the total healthcare market in the next 5 years, up from 15% today. However, this is still significantly below the benchmark set by developed markets. “Both the per capita consumption of medical devices and the ratio of device spending to total healthcare expenditure in China is still far below developed markets such as Japan and the US. This indicates significant potential in the long run,” confirms Su Hua, Principal, IMS Consulting Group. A “New Normal” As the market grows, however, so too does attention from the government, which has recently announced a number of new policies meant to enhance oversight and regulation of medical device registration, pricing, and distribution, among others. Such policies are expected to have enormous impact on domestic and multinational (MNC) firms alike and will certainly result in a “new normal” characterized by greater regulation, and slower growth; Over the next few years, the market is expected to dip to a CAGR of 14% after years of robust 19% growth (Fig. 1). Significantly, many of these shifts in policy are for the benefit of local firms, highlighting China’s clear intention to encourage, and continuously support, the expansion of domestic products’ market share. This is especially +19% +14% 20 2011 34 2014 24 2012 39 2015E 51 2017E 29 2013 45 2016E 58 2018E Fig. 1 China medical device market size Billion USD IMS China Market Prognosis, Sep.2015
- 22. 22 true at the provincial level, where recent announcements and purchasing agreements are clear signals of domestic favorability (Fig. 2). However, although the short term impact may be negative for MNCs, the policies discussed here will ultimately raise barriers to entry and encourage consolidation in the device sector — both of which are trends that will benefit large, innovative MNCs in the long term. Registration Many of the new policies for the medical device sector are focused on implementing a more regulated registration process (and one that is subsequently more accessible for local companies). “The reform of medical device registration is expected to accelerate local opportunity in specific sectors, and also to accelerate industry consolidation by ultimately eliminating companies without capital and technical strength” confirms Colin Yu, Engagement Manager, IMS Consulting Group. The following are three landmark decisions that are likely to have the greatest impact on medical device companies. These reforms will transform the competitive environment, especially for class I and II players. For innovators, the competition will intensify, as the barriers to entry are lowered, or even removed. For commodity players, these reforms represent a smoothing of the approval process, making it faster and easier to secure registration for class I and II devices. For class III products, however, registration will become significantly more time consuming, more expensive, and will necessitate investment in clinical trials; here, innovators surely have the advantage. 1. Authority is delegated to municipal and/or provincial levels The authority to approve all classes of medical devices has historically resided at the national level. Now, however, domestic devices in class I and II can be approved at the municipal or provincial level where there is a more favorable and supportive environment (imported products must still go through national-level approval). 2. Registration time and cost has increased for innovators The length of time needed to gain approval can often be prohibitively long, especially for new and innovative (often class III) products, or for companies that rely on being first-to-market. Quickly introducing innovative products will be extremely challenging. MNCs and innovators with greater technology capabilities and more capital will win (Fig. 3). 3. Clinical trial requirements have changed Clinical trial exemptions can now be granted to local class II devices, further increasing the efficiency and speed of the registration process for domestic companies. Imported class II devices, while also eligible for clinical trial exemption, must produce data from the original country. For class III Medical devices in China are monitored by the State Food and Drug Administration (SFDA) and organized into three classes (I, II and III). The rubric for classification evaluates products on a spectrum of risk to patients; the more potential risk to the patient, or the more oversight necessary for safe and effective use, the higher the classification. Sichuan province 2014 Increase local players' competitiveness — Domestic products now enjoy extra scores in tendering and favorable tax policies to domestic products. Jiangsu province 2014 Require hospitals to purchase locally-manufactured products — Province now equipped for pilot hospitals to purchase local products such as CT. Zhejiang province 2014 Require hospitals to purchase locally-manufactured devices — Now mandatory with the first domestic nuclear magnetic resonance (NMR). Hubei province 2014 Support local innovative products — Province is now equipped with 120 sets of local, innovative medical devices in 60 pilot medical facilities. Guangdong province 2014 Provincial tenders policy tilt to local — Shenzhen, Dongguan now exclude imported products in provincial tenders. Fig. 2 Provincial policies favoring local players Fig. 3 Increased registration cost for class III MNC devices ~ 300K AfterBefore 3~5K currency: RMB Source: Policy review, industry expert interview, IMS Health analysis
- 23. 23 devices, however, new requirements for clinical trials will further elevate the cost to gain market entry. In all, the medical device market, in terms of registration, will now closely resemble the larger pharma market, especially for MNCs. Market Access In this “new normal” environment, MNC and local manufacturers will both face dramatically increasing pricing pressures, the result of new tendering policies that will significantly cut prices, limit opportunities and force an entirely new approach to the price-volume tradeoff. Tendering In many ways, the practice of tendering in the medical device sector resembles that of the pharma industry 10-15 years ago. Hampered by an irrelevant evaluation structure and a challenging reference system, the tendering process poses enormous challenges for local and MNC device manufacturers alike. A new policy of extending reference pricing to the regional or national level will be especially challenging; prices will be set according to the lowest price in the country, rather than in a smaller, more relevant area. Such regulations have the potential to cut prices by 20-30%, especially in larger cities. Distribution Distribution has historically been a costly and corrupt system in the medical device sector, primarily because high-value medical device distributors are often also responsible for a wide range of services along the value chain, notably sales and marketing. And while their precise role can vary depending on size, the markup imposed on products to cover such activities can be astonishingly high, and rarely consistent or substantiated. The subsequent opportunity for corruption is, unsurprisingly, widespread, driving retail prices that are several times higher than ex-factory prices. In order to regulate these margins and business behaviors, and ultimately to protect patients from bearing the costly burden of such high retail prices, the government has proposed a series of strict margin caps. For many companies, particularly MNCs who often suffer from low price ceilings, such regulations will necessitate significant adjustments to pricing, partnership and distribution strategies. Responding to the “New Normal” For many MNCs, the policy shifts discussed here have resulted in decreased growth and restricted market access. Medtronic, for example, has experienced a slowdown over the past 2 years due in large part to tendering, price pressures, and anti-corruption policies. Similarly, BioMerieux’s growth decelerated under intense competition due to healthcare reforms and prolonged registration processes for portfolio updates. Both of these companies needed to take swift strategic action to mitigate the long term impact of new policies. For other MNCs, there are several options to consider in order to build a more stable financial and commercial strategy in this environment: 1. Refine pipelines: Higher barriers of entry for Class III products can be an advantage. Prioritize pipelines to focus on more innovative products and be prepared for longer registration timelines. 2. Consider M&A: Mergers, acquisitions, or other local partnerships can broaden product portfolios and mitigate risk. 3. Localize: Localizing production, or forming a joint venture with local manufacturers can reduce costs and achieve a valuable local production label, both of which are necessary to stay ahead of new pricing policies. 4. Differentiate: Launching products with innovative features that better serve physicians’ needs can significantly increase bargaining powers in tendering. 5. Improve compliance: New commercial models can proactively address current distribution pressures, and leverage networks to remain up to date on regulations. Of course the right course of action will vary by company, and will depend on a variety of factors, including appetite for risk, size, capital and more. Nevertheless, these recommendations signify a critical call to action for MNCs — adjust your business model, and your capabilities, to fit the “new normal,” or risk losing valuable market share and future opportunities. To continue the conversation, please contact: Hua Su, Principal, IMS Health China at hsu@cn.imshealth.com and Colin Yu, Engagement Manager, IMS Health China at zlyu@cn.imshealth.com
- 24. 424840eropagniS,-12#teertSssorC8•ewr/moc.htlaehsmi.www•moc.htlaehsmi@ofniEWR•SNOITULOSECNEDIVEDLROW-LAERHTLAEHSMI 01/02/03/04 6 Asia Pacific +65 6412 7365 • USA +1 (703) 992 1025 • Europe +44 (0) 20 3075 4800 • Latin America +52 55 5089 5205 Technology- Enabled Analytics Real-World Data (RWD) Services and Engagement IMS HEALTH REAL-WORLD EVIDENCE SOLUTIONS Accesstherightreal-worldda ta Analyzethedata Create datasets Apply the insights Uncovering insights from real-world data to improve decision making and patient outcomes Your Partner of Choice for Building an RWE Ecosystem IMS Health helps clients establish RWE ecosystems to support product value, safety, access and pricing strategies for the entire organization. Let us show how we can help you capture value from solutions with the right data, advanced analytics and technology: • 500+ million anonymous longitudinal patient data records including EMR, claims data, hospital data and lab tests in 25+ markets • Partnerships and data sourcing capabilities to get the data that is right for your needs • Leading edge technology and analytics to enhance your understanding of patient outcomes, healthcare costs, pharmacoepidemiology, drug safety and product value • Experts in 20+ markets with deep expertise in RWE, HTA and payer requirements to translate insights into actions • 3,000+ publications building healthcare knowledge • Asia Pacific hubs in Singapore, China and Japan to service local evidence needs
