Application of centrifugation and Spectrophotometry
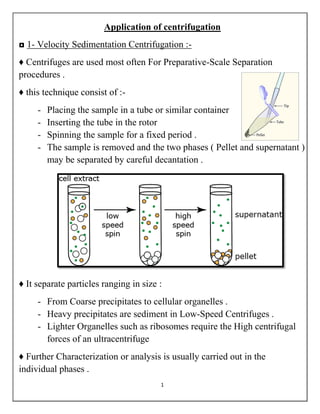
- 1. 1 Application of centrifugation ◘ 1- Velocity Sedimentation Centrifugation :- ♦ Centrifuges are used most often For Preparative-Scale Separation procedures . ♦ this technique consist of :- - Placing the sample in a tube or similar container - Inserting the tube in the rotor - Spinning the sample for a fixed period . - The sample is removed and the two phases ( Pellet and supernatant ) may be separated by careful decantation . ♦ It separate particles ranging in size : - From Coarse precipitates to cellular organelles . - Heavy precipitates are sediment in Low-Speed Centrifuges . - Lighter Organelles such as ribosomes require the High centrifugal forces of an ultracentrifuge ♦ Further Characterization or analysis is usually carried out in the individual phases .
- 2. 2 ◘ 1- Fractional Centrifugation : ♦ Much of our current understanding of cell structure and function depends on separation of subcellular components by centrifugation . ♦ This specific method of separation consists of successive centrifugation at increasing rotor speeds . ♦ the rotor chamber must be kept at low temperature to maintain the native structure and function of each cellular organelle ant its component biomolecules . ♦ The Operation :- - A high-speed centrifuge equipped with a fixed angle rotor is the most appropriate for the first two centrifugations at 600 xg and 20,000 xg . - After each centrifuge run , the supernatant is poured into another centrifuge tube , which is then rotated at the next higher speed . - The final centrifugation at 100,000 xg to sediment microsomes and ribosomes must be done in an ultracentrifuge . - The 100,000 xg supernatant , the cytosol , is the soluble portion of the cell and consists of soluble proteins and smaller molecules.
- 3. 3 Spectrophotometry ◘ The instruments that measure electromagnetic radiation have several concepts and components in common . ◘ Photometric instruments measure light intensity without consideration of wavelength . ◘ Most instruments today use :- - filter (photometers) - prisms , gratings ( spectrometers ) ♠ to isolate or select a narrow range of the incident wavelength . ◘ Radiant energy that passes through an object will be partially reflected , absorbed , and transmitted .
- 4. 4 ◘ Electromagnetic radiation : is described as photons of energy travelling in waves . ◘ The relationship between wavelength and energy E is described by Planck’s formula : - Because the frequency of a wave is inversely proportional to the wavelength .., the energy of electromagnetic radiation is inversely proportional to wavelength . ◘ Electromagnetic radiation includes a spectrum of energy from ( short- wavelength , high energetic x-rays & γ-rays ) to ( long-wavelength Radio- frequencies ) ◘ Visible light falls in between , the color violet at 400 nm and red at 700 nm wavelengths being the approximate limits of the visible spectrum .
- 5. 5 ♣ Absorption & Emission Spectra : • Instruments measure either absorption or emission of radiant energy to determine concentration of atoms or molecules . • The two phenomena , absorption and emission , are closely related . • For a ray of electromagnetic radiation to be absorbed , it must have the same frequency as a rotational or vibrational frequency in the atom or molecule that it strikes ( hits ) . • levels of energy that are absorbed move in discrete steps and any particular types of molecule or atom will absorb only certain energies and not others . • when energy is absorbed valence electrons move to an orbital with a higher energy level . • Following energy absorption , the excited electron will fall back to the ground state by emitting a discrete amount of energy in the form of a characteristic wavelength of radiant energy . • Absorption or emission of energy by atoms results in a line spectrum .
- 6. 6 • Because of the relative complexity of molecules , they absorb or emit a bank of energy over a large region . • light emitted by incandescent solids ( tungsten or deuterium ) is in continuum .
- 7. 7 ◘ Principle :- ○ Beer’s Law :- ♦ Definition : - The relationship between absorption of light by a solution and the concentration of that solution . - States that the conc. Of the a substance is directly proportional to the amount of light absorbed or inversely proportional to the logarithm of the transmitted light . ♦ when a beam of monochromatic light entering a solution , some of the light is absorbed and the remainder passes through , strikes a light detector . - The detector convert the light to an electric signal .
- 8. 8 ♦ Percent Transmittance ( % T ) :- - Is the ratio of the radiant energy transmitted ( I ) divided by the radiant energy incident on the sample ( Io ) - All light absorbed or blocked results in 0% (T) transmittance . - A level of 100% T is obtained if no light is absorbed . - In practice , the solvent without the constituent of interest is placed in the light path , most of the light is transmitted , but a small amount is absorbed by the solvent and cuvet or is reflected away from the detector . - The electrical readout of the instrument is set arbitrarily at 100% T, while the light is passing through a “blank” or reference . - The sample containing absorbing molecules to be measured is placed in the light path . - The difference in amount of light transmitted by the blank and that transmitted by the sample is due only to the presence of the compound being measured .
- 9. 9 - The % T measured as : the ratio of the sample transmitted beam divided by the blank transmitted beam . - %T = sample beam signal / blank beam signal ×100 ♦ Absorbance :- • Is the amount of light absorbed . • It cannot be measured directly by a spectrophotometer . • It is mathematically derived from %T as follow :- %T = ( I/Io) ×100 A = - log ( I/Io) A = log ( 100%) – log %T A = 2-log %T - Where : ( Io) is incident light & ( I ) is Transmitted light . • According to beer’s law :- Absorbance is directly proportional to concentration . A = ɛ*l*c - Where ( ɛ ) is the molar absorptivity , the fraction of a specific wavelength of light absorbed by a given type of molecule . - ( l ) is the length of light path through the solution . - ( c) is the concentration of absorbing molecules .
- 10. 10 ♦ Absorptivity : • Depends on molecular structure and the way in which the absorbing molecules react with different energies . • For any particular molecular type , absorptivity changes as wavelength of radiation changes . • The amount of light absorbed at a particular wavelength depends on the molecular and ion types present and may vary with , PH and temperature. • because the path length and molar absorptivity are constant for a given wavelength , C . ♦ Unknown concentration are determined from a calibration curve that plots absorbance at a specific wavelength versus concentration for standards of known concentration . • For calibration curves that are linear and have a zero Y-intercept , unknown concentration can be determined from a single calibrator . • Beer’s Law deviations : - Not all calibration curves result in straight lines . - Deviation from linearity are typically observed at high absorbance . - The stray light within an instrument will ultimately limit the maximum absorbance that a spectrophotometer can achieve , typically 2.0 absorbance units .
- 11. 11 ♦ Beer’s law Examples : %T = ( I/Io) ×100 A = - log ( I/Io) A = log ( 100%) – log %T A = 2-log %T A = ɛ*l*c (ε ): (Greek letter, epsilon) is the molar absorptivity of the solute with units of M-1 cm-1 (or ( L mol-1 cm-1 or mol-1 dm3 cm-1 ) (l) is the path length of the light through the solution in units of cm. (C) is the concentration of the solution in mol L-1 (or mol dm-3 or M ) ◘ Using a cuvette with a length of 1 cm, you measured the absorbance of a solution with a concentration of 0.05 mol/L. The absorbance at a wavelength of 280 nm was 1.5. What is the molar absorptivity of this solution? ɛ280 = A/lc = 1.5/(1 x 0.05) = 30 L mol-1 cm-1 ◘ A solution of Tryptophan has an absorbance at 280 nm of 0.54 in a 0.5 cm length cuvette. Given the absorbance coefficient of Tryptophan is 6.4 × 103 LMol-1 cm-1 . What is the concentration of solution? Solution: As : ε = A / l c l = 0.5 cm A= 0.54 ε = 6.4 × 103 LMol-1 cm-1 C=? So c = A/ε l = 0.54 / 6.4 × 103 × 0.5 Answer = 0.000168 M ◘ A solution of thickness 2 cm transmits 40% incident light. Calculate the concentration of the solution, given that ε = 6000 dm3 /mol/cm. Solution: A = 2 - log %T = 2 – log 40 = 2 – 1.6020 = 0.398 A = ε l c l= 2cm ε = 6000 dm3 /mol/cm A=0.398 c=? So c = A/ ε l = 0.398/ 6000 × 2 Answer = 3.316 X 10 – 5 mol / dm3
- 12. 12 ◘ A solution shows a transmittance of 20%, when taken in a cell of 2.5 cm thickness. Calculate its concentration, if the molar absorption coefficient is 12000 dm3 /mol/cm. Solution: A = 2 – log %T = 2 - log 20 = 2 – 1.301 = 0.698 A = ε l c l= 2.5 cm ε = 12000 dm3 /mol/cm A=0.698 c=? So c = A/ ε l = 0.698/ 12000 × 2.5 Answer = 2.33 X 10 – 5 mol / dm3 ◘ Calculate the molar absorptivity of a 1 x 10-4 M solution, which has an absorbance of 0.20, when the path length is 2.5 cm. Solution: A = ε l c l= 2.5 cm A= 0.20 C= 1 x 10 – 4 M ε =? So ε = A / l c = 0.20/2.5 ×1×10-4 Answer = 800 dm3 /mol/cm ◘ Calculate the molar absorptivity of a 0.5 x 10-3 M solution, which has an absorbance of 0.17, when the path length is 1.3 cm. Solution: A = ε l c l= 1.3 cm A= 0.17 C= 0.5 x 10-3 M ε =? So ε = A / l c = 0.17/ 1.3 × 0.5 x 10-3 Answer = 261.53 dm3/mol/cm.
- 13. 13 ◘ A CaCO3 solution shows a transmittance of 90%, when taken in a cell of 1.9 cm thickness. Calculate its concentration, if the molar absorption coefficient is 9000 dm3 /mol/cm. Solution: A = 2 - log %T = 2 - log 90 = 2 – 1.954 = 0.045 A = ε l c l= 1.9 cm ε = 9000 dm3/mol/cm A=0.045 c=? So c = A/ ε l = 0.045/ 9000 × 1.9 Answer = 2.631 × 10 -6 mol / dm3 ◘ A 1.00 × 10–4 M solution of an analyte is placed in a sample cell with a path length of 1.00 cm. When measured at a wavelength of 350 nm, the solution’s absorbance is 0.139. What is the analyte’s molar absorptivity at this wavelength? l = 1.00 cm c = 1.00 × 10–4 M A=0.139 ε =? So ε = A / l c = 0.139/ 1.0 × 1.00 x 10-4 Answer = 1390 cm−1 M−1 Spectrophotometry
