Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Unsaturated, saturated and supersaturated solutions

Unsaturated, saturated and supersaturated solutions
Similar to 00.pure substances vs. mixtures presentation
Similar to 00.pure substances vs. mixtures presentation (17)
Salt dissolving in water physical or chemical Def.pdf

Salt dissolving in water physical or chemical Def.pdf
More from a arg
More from a arg (20)
Estructura tierra y tectonica placas_ies isabel buendía

Estructura tierra y tectonica placas_ies isabel buendía
Metazoos: reproducción y desarrollo de J.I.Noriega

Metazoos: reproducción y desarrollo de J.I.Noriega
00.pure substances vs. mixtures presentation
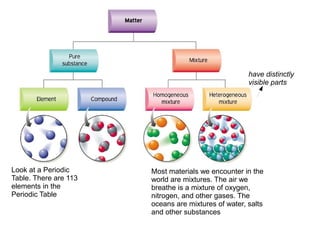
- 1. have distinctly visible parts Look at a Periodic Most materials we encounter in the Table. There are 113 world are mixtures. The air we elements in the breathe is a mixture of oxygen, Periodic Table nitrogen, and other gases. The oceans are mixtures of water, salts and other substances
- 2. A pure substance has a definite and constant composition — like salt or sugar. The composition of a pure substance doesn’t vary. An element is composed of a single kind of atom. A compound is composed of two or more Chemists CAN’T separate elements in a specific ratio. For example, water is a compound made up the components of a of two elements, hydrogen (H) and oxygen compound AT ALL. (O). Chemists can’t easily separate the components of a compound: They have to resort to some type of chemical reaction.
- 3. the different parts of a mixture can be easily separated by physical means, such as filtration. For example, suppose you have a mixture of salt and sand, and you want to purify the sand by removing the salt. You can do this by adding water, dissolving Mixtures are physical the salt, and then filtering the combinations of pure substances mixture. You then that have no definite or end up with pure constant composition — the sand. composition of a mixture varies according to who prepares the mixture.
