FDC Ban - What's Right and What's Wrong?
- 1. u 46 u march 28 -april 10, 2016 Business India u the maga zine of the cor por ate wor ldFocus T hose familiar Shakespeare’s Julius Caesar would have dubbed it the “Ides of March” for the Indian pharmaceutical indus- try. But according to the Central gov- ernment gazette dated 10 March, 344 medicinal formulations repre- senting a total sales value of almost H3,800 crore, was on the cards for the past several months. When it actually became public knowledge about a week later, the notifica- tion predictably caused “shock and awe” throughout the Indian pharma industry. Stock prices of major com- panies plunged and industry leaders went into a tizzy. About 20 companies have obtained an interim stay from the Delhi High Court and another 80 are getting ready to follow suit. “We are planning our own legal action as an industry body,” says S.V. Veerramani, national president, Indian Drug Manufacturers Association (idma). The government action was based on the report of a six-member com- mittee headed by Professor Chandra- kant Kokate, appointed in September 2014 to look into the issue of irra- tional medicines being sold in the market. They examined 6,214 drug formulations each of which com- prised two or more pharma ingredi- ents. These are known as Fixed Dose Combinations or fdcs. The Kokate Committee submit- ted its first interim report in April 2014 in which the products exam- ined were categorised as (A) Rational, (B) Irrational, (C) Requiring further deliberation and (D) Requiring sub- mission of additional data from the manufacturing companies. Among all the fdcs that the Kokate Com- mittee studied, it placed about 1,083 in category B and C, of which 344 were considered definitely irratio- nal and an immediate ban was rec- ommended for them. This report was submitted to the government on 10 February 2016. The remaining products, placed in Category C, are being discussed further and would be under the scanner of the Central Drugs Standards Control Organisa- tion (cdsco) for some time. Many industry watchers fear that the government may soon impose a similar ban on all 1,083 formulations, which could shrink the H100,000 crore Indian pharmaceutical mar- ket in India by as much as H12,000 crore. Besides, there are a large num- ber of products which were initially granted marketing permission by the various State Licensing Authori- ties (slas) many years ago and have been prescribed by doctors all over the country ever since. These are the Category D products for which the cdsco has now asked manufacturers to submit fresh scientific data within the next 18 months. Interestingly, ims Health, the larg- est market research agency to track the pharma market in the coun- try, may have underestimated the actual impact of the current ban. Its estimate of the sales impact of the recent ban order is based on just 215 medicinal formulations which it has been tracking, while another 129 are not even on its radar! Nitin Goel, Bad medicines The dispute could become a tortuous legal battle, unresolved for years
- 2. u 47 u march 28 -april 10, 2016 Business India u the maga zine of the cor por ate wor ld Focus managing director, ims Health India, was unavailable for comment. Though the Kokate Committee has listed its objection to each of the drugs it has catesgorised as irrational, indus- try leaders have criticised the Febru- ary report, saying that it does not take the industry’s viewpoint into consid- eration. “The Indian Pharmaceutical Alliance (ipa) neither supports ‘irra- tional’ fixed dose combinations nor seeks to defend them for profit. It is committed to providing its custom- ers with safe, effective and quality medicines. However, it has serious concerns about the accuracy of the database furnished to the expert committee; the processes followed by the expert committee; the prima facie violation of the terms of reference; the relevance of the experts to the task assigned; and the final decision process of banning the fdcs,” says ipa secretary general Dilip G. Shah. “The government order to ban 344 fdcs en bloc is arbitrary and unfair. Even though vague show causes notices without details were received in some cases, no oppor- tunity for a personal hearing has been given to any manufacturer. The many issues raised by manufacturers in reply to the notices have also not been answered by the government and it is clear that the department has not applied its mind to the mer- its of each case when issuing the en bloc order barring 344 fdcs in one go. This is also not in public interest as lakhs of patients will be suddenly denied essential, practically effec- tive and proven safe drugs fdcs pre- scribed by qualified doctors,” says a news release from the idma. Same language This is possibly because the gazette notification announcing the ban order has listed all the 344 medicinal formulations one by one, but used exactly the same language in justify- ing its action. In each case, the noti- fication reads as follows: S.O. 709(E).—Whereas, the Central Government is satisfied that the use of the drug fixed dose combination of Paracetamol + Cetirizine + Caffeine is likely to involve risk to human beings whereas safer alternatives to the said drug are available; And Whereas, the matter has been examined by an Expert Committee appointed by the Central Government and the said Expert Committee recommended to the Central Government that the said drug is found to have no thera- peutic justification; And Whereas on the basis of the recommendations of the said Expert Committee, the Cen- tral Government is satisfied that it is necessary and expedient in public interest to regulate by way of prohibi- tion of manufacture for sale, sale and distribution for human use of the said drug in the country; Now, there- fore, on the basis of the recommen- dations of the said Expert Committee and in exercise of powers conferred by section 26A of the Drugs and Cos- metics Act, 1940 (23 of 1940), the Central Government hereby pro- hibits the manufacture for sale, sale and distribution for human use of drug fixed dose combination of Par- acetamol + Cetirizine + Caffeine with immediate effect.” In the remaining sections of the 288-page gazette notification, only the chemical names of the medicines have been changed suitably but the language otherwise remains exactly the same. Similarly, in the Kokate Committee report, on which the entire government action is based, the column on industry responses for each of the banned drugs is iden- tical. It reads as follows: “The replies/ clarifications wherever available from firms and the earlier data sub- mitted by them were thoroughly examined. The Committee observed that the data submitted and the peer reviewed scientific evidences do not support the rationality of this fdc. Hence the committee considered this fdc to be irrational.” But not everyone associated with the pharmaceutical industry or the medicalprofessionsupportstheindus- try viewpoint. Tapan Ray, former president of the Organisation of Phar- maceutical Producers of India (oppi) and a veteran of the pharmaceuti- cal industry, writes in his blog (www. tapanray.in) “In June 2013, cdsco announced the ‘Policy guidelines for approval of Fixed Dose Combinations Veerramani: planning legal action
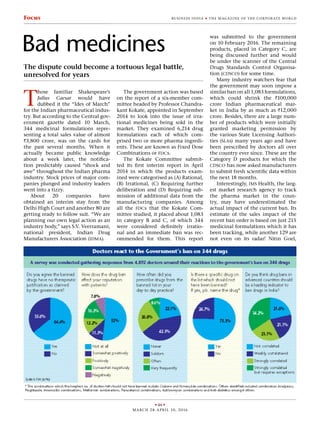
- 3. u 48 u march 28 -april 10, 2016 Business India u the maga zine of the cor por ate wor ldFocus (fdcs)’ in India. According to cdsco, just 1,193 fdcs were approved by the dcgi, since 1961 till November 2014. Thus, all drug manufactur- ers should clearly know, which fdc has been approved by the dcgi, and when, leaving no scope for any ambi- guity in this area. Thus, there should be no problem in total conformance to the above ‘fdc Policy Guidelines’ by these drug producers. In the same year – 2013, a public notice was also, reportedly, issued, calling all those drug players manufacturing fdcs to apply with the requisite fee, in the prescribed form to the dcgi office, providing the required details.” Only after that was the Kokate Committee appointed by the government. The rest, as they say, is history. “fdcs are a useful tool when they prescribed and marketed judiciously, but they should all be approved by the dcgi after proper trials. Just because the ingredients of an fdc have been approved separately does not mean the fdc can be assumed to be safe and effective,” says Dr K.K. Agrawal, honorary secretary general, Indian Medical Association (ima), the largest umbrella body of medical practitioners all over the country. Likewise, when eMediNexus, a healthcare information and advocacy Website conducted an impromptu survey of 4,892 clinical specialists from different parts of India last week, it was revealed that as many as 64 per cent of the respondents agreed with the government action! Further, approximately 52 per cent of doctors said their own reputa- tions (in their patients’ view) would not be affected at all by the govern- ment action, and about 64.6 per cent said they did not prescribe any of the drugs from the banned list. However when asked whether there was a spe- cific medicine which they felt should not have been banned, a sizeable number (75.3 per cent) answered in the affirmative. Codeine combina- tions which are prescribed almost universally for treatment of cough, and nimesulide preparations for pain and fever received the most votes in their favour. Other drugs that many doctors wished to continue pre- scribing included antibiotics like azithromycin, paracetamol (for fever) and some medicines for diabetes. Hard hit Anup Soans, editor of MedicinMan, a widely read electronic monthly news- letter focused on the Indian pharma- ceutical industry, had quite another explanation. “One has to exam- ine the timing of the government’s move, the fdc scrutiny and ban was on the cards for quite some time. Why now? One wonders whether it is intended to counter criticism of the Compulsory Licensing (cl) clause, because some of hardest hit are Pfizer and Abbott, both US compa- nies!” Compulsory Licensing, which is a provision under the Indian Pat- ent Act 2005, allows the government to force any multinational pharma- ceutical company to share its patent rights with an Indian company if it is required for the welfare of the Indian people. This has long been a bone of contention in India’s dealings with western countries and recent reports in the global media suggested that India had promised the US not to invoke the clause, causing much heartburn in certain quarters. How- ever it cannot be denied that other than Pfizer and Abbott, most of the companies affected by the latest gov- ernment order are of Indian origin. Likewise Medecins Sans Frontieres (msf), also known as Doctors Without Borders, issued a statement welcom- ing the ban order saying, “The list of banned drugs includes several fdcs containing multiple antibiotics long used injudiciously, contributing to the development of resistant strains of infection-causing bacteria. Irrational fdcs containing important anti-tb drugs such as quinolones (ofloxacin and levofloxacin) and linezolid are rampantly available in the Indian pri- vate market, which has resulted in worsening prevalence of drug-resis- tant tuberculosis (dr-tb). “This year, India has a double chal- lenge: phase out irrational antibiotic fdcs in the private sector that are con- tributing to resistance generation, and to phase in the who-recommended daily fdc regimen for drug-sensitive tb (ds-tb) to the national tb program, which has been shown to increase adherence and reduce pill burden.” What happens next is anybody’s guess.TheDelhiHighCourthasissued an interim stay on five banned prod- ucts of Abbott and some products of the other companies and listed their petitions for hearing next week. Peti- tions no: 2213/2016 and 2214/2016 (Abbott) have been listed as ‘pending’ just like petitions no. 2395/2016 and 2212/2016 (Pfizer). At least 20 other companies have also obtained a stay on their own products, while idma is planning its own legal action. As it happened in the case of the government’s 2007 effort to weed out irrational drug combinations, this time too the dispute could become a tortuous legal battle, unresolved for years on end. In such a case, the ulti- mate loser would be the Indian peo- ple. One must hope and pray that this does not happen. u SUMIT GHOSHAL feedback@businessindiagroup.com
