Early Discoveries of the Electron and Atomic Structure
•Download as PPT, PDF•
1 like•368 views
JJ Thomson discovered the electron through his work with cathode ray tubes in the late 1800s. This led him to propose the plum pudding model of the atom, with negatively charged electrons embedded in a positively charged medium. Robert Millikan later directly measured the charge of individual electrons through his oil drop experiment in 1905, enabling him to calculate the electron's mass. Ernest Rutherford then used the gold foil experiment to discover the nucleus as the small, dense, positively charged center of atoms through deflections of alpha particles in 1910.
Report
Share
Report
Share
More Related Content
What's hot
What's hot (20)
Viewers also liked
Viewers also liked (7)
Similar to Early Discoveries of the Electron and Atomic Structure
Similar to Early Discoveries of the Electron and Atomic Structure (20)
Atomic Structure ememememememmememeeemmemememememem

Atomic Structure ememememememmememeeemmemememememem
Atomic Structure ememememememmememeeemmemememememem

Atomic Structure ememememememmememeeemmemememememem
Historical Development of the Atom model of an atom.ppt

Historical Development of the Atom model of an atom.ppt
Lesson 4 Not Indivisible (The Structure of the Atom)

Lesson 4 Not Indivisible (The Structure of the Atom)
More from Chris Hitchens
More from Chris Hitchens (20)
Ch4 lesson 5 electron notation configuration_theory

Ch4 lesson 5 electron notation configuration_theory
Early Discoveries of the Electron and Atomic Structure
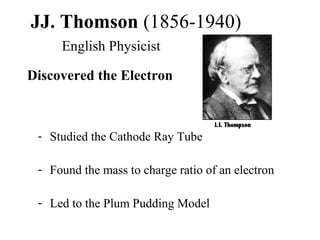
- 1. JJ. Thomson (1856-1940) English Physicist Discovered the Electron - Studied the Cathode Ray Tube - Found the mass to charge ratio of an electron - Led to the Plum Pudding Model
- 2. The Design of the Cathode Ray Tube
- 3. So! Thompson has discovered the electron, and it must live inside atoms. It is much less massive than the the atom itself, so perhaps we have little electrons stuffed into the ‘rest’ of the atom like raisins in the oatmeal, or: Plum Pudding...
- 4. Robert Milliken (1868-1953) American Physicist Performed the Oil Drop Experiment – Measured minimum electric charge that could be carried by a particle (1905) – This was the charge of the electron – Enabled him to find the mass of the electron
- 5. Millikan’s Oil Drop Experiment Used To Determine the Charge of an Electron
- 6. Ernest Rutherford (1871-1953) • New Zealand born physicist • Worked with nuclear radiation • Performed the Gold Foil Experiment • Discovered the nucleus (1910) • Discovered the proton (1918)
- 7. The Gold Foil Experiment Demonstrated that the atom contains a nucleus which is: a. Very tiny – most of the particles missed b. Positively charged – some were deflected c. Very dense – some bounced off
- 8. James Chadwick (1891-1974) • Discovered the neutron in 1932 • In college he stood in the wrong line. He wanted to be a mathematician, but was in the line for physics and was too embarrassed to switch. • Instead he became a famous physicist.
- 9. End! The story’s not over, but we’ll pick it up in chapter 4.
