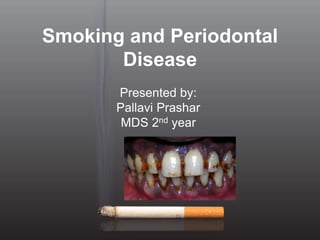
Smoking and periodontal disease
- 1. Smoking and Periodontal Disease Presented by: Pallavi Prashar MDS 2nd year
- 2. Introduction • Smoking is associated with a wide spectrum of disease including stroke, coronary artery disease, peripheral artery disease, gastric ulcer, and cancers of the mouth, larynx, esophagus, pancreas, bladder, and uterine cervix. • It is also a major cause of chronic obstructive pulmonary disease and a risk factor for low birth weight babies. • Approximately 50% of regular smokers are killed by their habit and smoking causes 30% of cancer deaths.
- 3. Smoking Epidemic United States • In 1993 25.4% of population smoked – 27% of men – 24% of women • In 2007, this decreased to 20.8% – 23.9% of men – 18% of women • As per NHANES III – During period 1988 to 1994 • 27.9% of adults were current smokers • 23.3% of adults were former smokers • Smoking was higher in younger age group (34 years) than in older age group (55 years)
- 4. European Union • An average of 29% of the adult population smoke, ranging from 17.5% in Sweden to 45% in Greece. • Most smokers start the habit as teenagers, with the highest prevalence in the 20–24- year-old age group. • Socioeconomic differences also exist with higher smoking in the lower socioeconomic groups.
- 5. India
- 7. Different methods of tobacco smoking Pipes
- 8. Classification of smokers Heavy smokers 20 / day Light smokers 19 / day Current Smoker Who have smoked 100 cigarettes in their life time and currently smokes Former Smoker Who have smoked 100 cigarettes in their life time and do not currently smokes Non Smoker Who have not smoked 100 cigarettes in their life time and do not currently smokes
- 9. • Many smokers are trying to quit and therefore, simply asking how many cigarettes they are smoking today may not give an accurate assessment of their life time exposure. • To get around this problem Pack Years should be calculated as follows: Pack Years = (no. of packs smoked/day) X (no. of years of smoking)
- 10. Constituent Of Tobacco Smoke • Cigarette smoke is a very complex mixture of substances with over 4000 known constituents and comprises of: Gasseous phase Carbon monoxide Ammonia Formaldehyde Hydrogen Cyanide Acrolein Benzopyrene Dimethylnitrosamine Solid (particulate) phase Nicotine Tar Benzene Benzopyrene
- 12. • Tar – In its condensate form is sticky brown substance that stains fingers and teeth yellow brown. • Nicotine – Alkaloid found within tobacco leave and evaporates when the cigarette is lighted. – It is quickly absorbed and reaches the brain within 10-19 secs. – Nicotine in tobacco smoke from most cigarettes is not well absorbed through the oral mucosa because the nicotine is in an ionized form as a result of the pH (5.5). In contrast cigar and pipe smoke is more alkaline (pH 8.5), which allows good absorption of un-ionized nicotine through the buccal mucosa (Benowitz 1988). – Nicotine is absorbed rapidly in the lung where the smoke is well buffered. – It is highly addictive and causes rise in BP, Increased heart rate and respiratory rate, and peripheral vasoconstriction. Tar and nicotine yields reduced due to use of filters Dose of tobacco intake – depends on the way an individual smokes (Benowitz 1989)
- 13. Assessment of smoking status • The patient’s exposure to tobacco smoke can be measured in a number of ways, including interviewing the subject using simple questions or more sophisticated questionnaires and biochemical analyses (Scott et al. 2001). • Biochemical analyses: – Exhaled carbon monoxide in the breath, which is commonly measured in smoking cessation clinics, and – Cotinine (a metabolite of nicotine) in saliva, plasma/serum or urine
- 14. • Cotinine measurements are more reliable in determining a subject’s exposure to tobacco smoke because – the half-life is 14–20 hours compared with the shorter half-life of nicotine which is 2–3 hours (Jarvis et al. 1988). • The mean plasma and salivary cotinine concentrations of – regular smokers are approximately 300 ng/ml and urine concentrations are about 1500 ng/ml. – Non-smokers typically have plasma/saliva concentrations under 2 ng/ml, but this may be raised slightly due to environmental exposure (passive smoking).
- 15. Tobacco smoking Plaque and oral flora Periodontal tissues Homeostasis and healing potential Periodontal therapy Immune response
- 16. EFFECTS OF SMOKING ON PREVALENCE AND SEVERITY OF PERIODONTAL DISEASE Effect of smoking on gingiva Changes in the epithelium - hyperkeratotic, hyperplastic Greyish discoloration of the gingiva Increased amounts of IL-1, IL-6 and PGE2 (Johnson et al 1996)
- 17. Smoking – predisposing factor for ANUG use of tobacco frequency of ANUG (Rowland 1999) Reason: Tar in the smoke irritating effect on gingiva Nicotine vasoconstriction of capillaries (Lindeboom 2005)
- 19. SMOKING influence the tissue response to irritation. activates the release of epinephrine promotes contraction of peripheral vessels reducing blood flow to the gingiva loss of vitality to the gingival epithelium onset of ANUG .{Karadachi et al}
- 20. Effect of smoking on gingival blood flow Transient decrease (Clarke et al 1981) the infusion of nicotine resulted in a transient decrease in gingival blood flow in a rabbit model. Transient increase (Baab & Oberg 1987) using laser Doppler flowmetry to monitor relative gingival flow in 12 young smokers, observed an immediate but transient increase in relative gingival blood flow during smoking, compared to the presmoking or resting measurements.
- 21. • The authors hypothesized that the steep rise in heart rate and blood pressure due to smoking could lead to an increase in the gingival circulation during smoking. • These results were confirmed by Meekin et al. (2000) who showed that subjects who smoked only very occasionally experienced an increase in blood flow to the head, whereas regular smokers showed no change in blood flow, demonstrating tolerance in the regular smoker. • Morozumi et al. (2004) showed that the gingival blood flow significantly increased at 3 days following quitting
- 22. Gingival inflammation and bleeding Smokers experienced less gingival bleeding (Bergstrom & Floderus- Myrhed 1983) NHANES III : Dose–response effect (Dietrich et al 2004) Quit-smoking program – improvement in parameters (Nair et al 2003) Rapid recovery of the inflammatory response
- 23. Periodontitis • Pindborg (1947) was one of the first investigators to study the relationship between smoking and periodontal disease. • Increased prevalence and severity of periodontal destruction. – On average, smokers are 4 times likely to have periodontal disease as compared to person who had never smoked. – Former smoker were 1.7 times more likely to have periodontitis than person who had never smoked – Older adults are approximatelty 3 times more likely to have severe periodontal disease and number of pack years of tobacco use is significant factor in tooth loss, coronal root caries and periodontal disease. – Cigarette smoking is associated with increased severity of generalized aggressive periodontitis I young adults. – Smokers between the age of 19-30 years are 3.8 times more likely to have periodontitis than non smoker.
- 24. • Deeper probing depths and a larger number of deep pockets (Feldman et al. 1983; Bergstrom & Eliassson 1987a; Bergstrom et al. 2000a) • More attachment loss including more gingival recession (Grossi et al. 1994; Linden & Mullally 1994; Haffajee & Socransky 2001a) – Smokers are more than 6 times as likely as non smokers to demonstrate continued attachment loss. – The prevalence of moderate and severe periodontitis and percentage of teeth with 5mm is more severe in current cigarette smoker. – Cigar and pipe smoker showed a severity of disease intermediate b/w the current cigarette smokeer and non smoker.
- 25. • More alveolar bone loss (Bergstrom & Floderus Myhred 1983; Bergstrom & Eliasson 1987b; Feldman et al. 1987; Bergstrom et al. 1991, 2000b; Grossi et al. 1995) – Over a 10 year period bone loss has been reported to be twice as rapid in smokers as in non smokers. – Proceeds more rapidly even in presence of excellent plaque control. • More tooth loss (Osterberg & Mellstrom 1986; Krall et al. 1997) – Young individuals smoking more than 155 cigarettes/day shows highest rate of tooth loss (Holm G,1994) – Tooth loss is increase in cigar and pipe smoker as compared to non smoker.
- 26. • Increased prevalence of periodontal disease with increased number of cigarettes smoked/day. • Decreased prevelance and severity with smoking cesation – Risk of periodontitis decreases with the increasing number of years since quitting smoking – Negative effects of smoking on host are reversible with smoking cesation and therefore, smoking ceastion should be integral part of periodontal education and therapy.
- 27. Effects of Smoking on Etiology and Pathogenesis of Periodontal Disease • The increased prevalence and severity of periodontal destruction associated with smoking suggests that the host- bacterial interactions normally seen in chronic periodontitis are altered, resulting in more aggressive periodontal breakdown. • This imbalance between bacterial challenge and host response may be caused by – changes in the composition of the subgingival plaque, – with increases in the numbers and virulence of pathogenic organisms, – changes in the host response to the bacterial challenge, – or a combination of both.
- 28. Microbiology • Smokers may have higher levels of plaque than nonsmokers, which may be accounted for by poorer levels of oral hygiene rather than higher rates of supragingival plaque growth (Bergstrom 1981; Bergstrom & Preber 1986). • Several studies have shown that smokers harbor more microbial species which are associated with periodontitis than non-smokers, including – P. gingivalis, – A. actinomycetemcomitans, – Tanerella forsythia (Bacteroides forsythus) (Zambon et al. 1996), • Smokers are 2.3 times more likely to harbour T. Forsythia than non smokers and former smokers – P. intermedia, – Peptostreptococcus micros, – Fusobacterium nucleatum, Campylobacter rectus (van Winkelhoff et al. 2001), – Staphylococcus aureus, – Eschericia coli, and – Candida albicans (Kamma et al. 1999).
- 29. • Increased colonization of shallow periodontal pockets by periodontal pathogens. • Smokers may have a higher proportion of sites harboring these putative periodontal pathogens, in particular the palatal aspects of the maxillary teeth and the upper and lower incisor regions (Haffajee & Socransky 2001a,b). • In contrast several studies have failed to show differences in the bacterial species between smokers and non-smokers (Preber et al 1992; Darby et al. 2000; Bostrom et al. 2001; van der Velden et al. 2003).
- 30. Immune-inflammatory response Effects on PMN function • Smokers have an increased number of leukocytes in the systemic circulation (Sorenson et al. 2004), but fewer cells may migrate into the gingival crevice/pocket (Eichel & Shahrik 1969). • Studies in vitro have shown a direct inhibition of neutrophil and monocyte–macrophage defensive functions by high concentrations of nicotine that may be achieved in patients using smokeless tobacco (Pabst et al. 1995). • MacFarlane and co-workers (1992) demonstrated abnormal PMN phagocytosis associated with a high level of cigarette smoking.
- 31. • Neutrophil defects have been associated with an increased susceptibility to periodontitis, including cyclic neutropenia where there is a reduction in the number of neutrophils, and conditions such as leukocyte adhesion deficiency (LAD 1 and LAD 2), which may be responsible for cases of generalized prepubertal periodontitis as described by Page et al. (1983). • Neutrophils obtained from the peripheral blood, oral cavity, or saliva of smokers or exposed in vitro to whole tobacco smoke or nicotine have been shown to demonstrate functional alterations in – chemotaxis, – phagocytosis, and – the oxidative burst
- 32. Smoking and lymphocytes function • The leukocytosis observed in smokers results in increased numbers of circulating T and B lymphocytes. Chronic exposure to nicotine Impairment of antigen-mediated T cell signalling Inhibits antibody-forming cell responses Immunosuppression (Sopori et al 1998)
- 33. sIgG levels are reduced in smokers – IgG2 (Fredriksson 1999) Effects of cigarette smoking on serum IgA and IgM classes – controversial Smoking decreases salivary IgA IgE is greatly elevated in smokers Reduced antibody levels to periopathogens
- 34. Cytokines • Elevated levels of tumor necrosis factor alpha (TNF- α) have been demonstrated in the gingival crevicular fluid (GCF) of smokers, • Elevated levels of prostaglandin E2 (PGE2), neutrophil elastase, and matrix metalloproteinase-8 (MMP-8). • In vitro studies also have shown that exposure to nicotine increases the secretion of PGE2 by monocytes in response to lipopolysaccharide (LPS).
- 35. Higher levels of IL-8, lower levels of IL-4 (Giannopoulou et al 2003) IL-1 levels half of that found in non-smokers (Petropoulos et al 2004) IL-6, IL-1 - no significant differences (Bostrom et al 2000)
- 36. Other Factors Elastase, 2-macroglobulin and 1-anti-trypsin levels Lower concentrations in heavy smokers (Persson 2001) • Tobacco smoking has a chronic effect on the elevated levels of soluble ICAM (sICAM) and there is evidence that the subject may return to more normal levels after quitting smoking (Scott et al. 2000b; Palmer et al. 2002). • GCF the levels of sICAM are much lower in smokers despite very much higher serum levels than non-smokers (Fraser et al. 2001).
- 37. Physiology • ↓Gingival blood vessels with ↑inflammation • ↓GCF flow and bleeding on probing with ↑ inflammation • ↓Subgingival temperature • ↑Time needed to recover from local anesthesia • Oxygen concentration in healthy gingival tissue appears to be less in smokers than non smokers, although this condition is reversed in presence of moderate inflammation.
- 38. Gingival Crevicular Fluid GCF nicotine concentrations - 300 times that of plasma (20ng/ml) Lower resting GCF flow rate (Persson et al 1999) Reduced GCF flow Defense mechanism hampered Less flushing – removal of microbes and waste products Episode of smoking transient increase in GCF flow rate (McLaughlin et al 1993) Quit-smoking programme – flow rate greater at 5 days postquitting (Morozumi et al 2004)
- 39. gingival redness Fewer gingival vessels GCF Less bleeding sites Suppression of the normal inflammatory response (Bergstrom 1986)
- 40. EFFECTS OF SMOKING ON RESPONSE TO PERIODONTAL THERAPY Non surgical periodontal therapy • ↓Clinical response to scaling and root planing – Numerous studies have indicated that current smokers do not respond as well to periodontal therapy as non-smokers or former smokers. • ↓Reduction in pocket depth – pocket depth reduction is more effective in nonsmokers than in smokers after nonsurgical periodontal therapy (Phase I therapy), including oral hygiene instruction, scaling, and root planing – Average pocket reductions of 2.5 mm for nonsmokers and 1.9 mm for smokers were observed in pockets that averaged 7 mm before treatment, even though plaque scores were less than favorable. (Bergstorm ,1988)
- 41. • ↓Gain in clinical attachment levels – The poorer reductions in probing depths and gains in attachment level amount to a mean of approximately 0.5 mm. – Much of this may be due to less recession of the marginal tissues in smokers as there is less edema and more fibrosis in the gingiva. (Lindhe 5th edition) • ↓Negative impact of smoking with ↑level of plaque control. • When pockets persist in smokers and nonsmokers after therapy, adjunctive topical antimicrobial therapy can be used to try to resolve the remaining pocket depths. • When scaling and root planing are used in combination with topical subgingivally placed tetracycline fibers, subgingival minocycline gel, or subgingival metronidazole gel, smokers continue to show less pocket reduction than nonsmokers. • Response to non-surgical treatment may be seen merely as resolution of infl ammation and improvement of the epithelial attachment together with some formation of collagen.
- 42. Surgical therapy and Implants • ↓Pocket depth reduction after surgery – In a longitudinal comparative study of the effects of four different treatment modalities, including coronal scaling, root planing, modified Widman flap surgery, and osseous resection surgery, smokers consistently showed less pocket reduction and less gain in clinical attachment levels than nonsmokers or former smokers. (Kaldahl WB,1996) • ↑Deterioration of furcations after surgery • ↓Gain in clinical attachment levels, ↓ bone fill, ↓ pocket depth reduction after bone graft procedure ↑ recession, and ↑ membrane exposure after GTR • ↓Pocket depth reduction after DFDBA • Conflicting data on the impact of smoking on implant success. Implant failure is 2.5 times greater (Wilson 1999) • Smoking cessation should be recommended before implants.
- 43. Maintainence therapy • The detrimental effects of smoking on treatment outcomes appears to be long-lasting and independent of the frequency of maintenance therapy. • ↑Pocket depth during maintenance therapy • ↓Gain in clinical attachment levels • ↑Recurrent/refractory disease in smokers • ↑Need for re-treatment in smokers • ↑Need for antibiotics in smokers to control the negative effects of periodontal infection on surgical outcomes • ↑Tooth loss in smokers after surgical therapy
- 44. Effect of smoking cessation on periodontal disease outcome • The periodontal status of former smoker is intermediate to that of current smokers or non smoker and appears to be usually closer to that of non-smokers. • One who quits smoking had better response to periodontal treatment as compared to non quitters and failed quitters. • The benefits of smoking cessation on periodontium is likely to be mediated through various pathways such as: – Shift towards a less pathogenic microflora – Recovery of gingival micro-circulation – Improvement in aspects of the immune inflammatory responss
- 45. Smoking cessation • Guidelines for health care provider when giving cessation advice includes ‘five A’s’ which are: Brief Intervention Program ASK ADVISE ASSESSASSIST ARRANGE
- 46. • Ask – Ask the patient about their smoking status – This should be part of medical history • Advice – Advice smokers of the association between oral disease and smoking – Be informative, honest and helpful but not judgemental – The patients response to information will reveal their interest in quitting • Assess – Assess the patient’s interest and readiness to attempt smoking cessation. – Patients may not yet be in an action phase to quit smoking, which is why it is always important to make these assessment, every time you see the patient. • Assist – Assist the patient in their quit attempt • Arrange – Arrange follow up or referral to professional smoking cessation service
- 47. Pharmacotherapy • Nicotine withdrawal agents: – Nicotine containing – Non-nicotine containing Nicotine containing agents • These contains nicotine and deliver measured amount of nicotine so that gradually nicotine intake can be reduced • It does not contain any toxic product such as tar and CO in cigarette smoke. • These are available in following forms: – Patches: available in different doses and worn for 16-24 hrs/day – Lozenges and gums: available in different flavours, should be chewed slowly to allow the nicotine to be absorbed through the mouth – Nasal spray – Inhalator: plastic mouth piece with a supply of nicotine cartridge that fits to one end, nicotine is absorbed in mouth by drawing on it like cigarette
- 48. Non-nicotine containing agents • Bupropion – Success rate at 12 months is 20-30% – Used as an antidepressant at higher doses but is effective for smoking cessation at low doses. – It is usually prescr ibed starting 1-2 weeks before the quit date – Initially at 150 mg OD for 6 days, then 150 mg BD for &-9 weeks – Contra-indicated in patients with epilepsy, anorexia nervosa, bulimia, bipolar disorder or severe liver disease. – The most common side effects are insomnia (up to 30%), dry mouth (10-15%), headache (10%), nausea (10%), constipation (10%), and agitation (5-10%)
- 49. • Other methods – Intensive counselling – Motivational interviewing – Cognitive behavorial therapy – Hypnosis – Acupuncture
- 50. Conclusion As an environmental factor, smoking interacts with the host and the bacterial challenge, resulting in an increased susceptibility to periodontitis and poorer response to treatment. Recent guidance suggest that dental practices should assess the smoking status of patients and motivate smokers towards quitting.
