Case record...Neuromyelitis optica
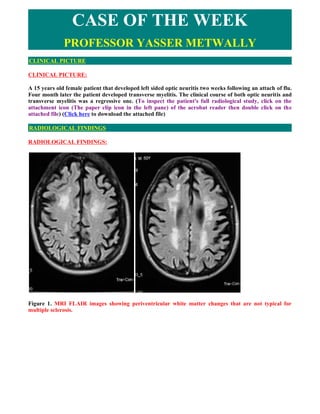
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE: A 15 years old female patient that developed left sided optic neuritis two weeks following an attach of flu. Four month later the patient developed transverse myelitis. The clinical course of both optic neuritis and transverse myelitis was a regressive one. (To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file) (Click here to download the attached file) RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS: Figure 1. MRI FLAIR images showing periventricular white matter changes that are not typical for multiple sclerosis.
- 2. Figure 2. MRI postcontrast T1 image (A) and MRI T2 image (B) showing a longitudinally extensive central hypointensity (A) and Hyperintensity (B) extending from C1 to D5. Notice the mild peripheral enhancement (A). Also notice the mild cord enlargement Figure 3. MRI T2 images showing cord enlargement, central hyperintensities involving more that 2/3 of the spinal cord in cross section and the central dot sign.
- 3. Figure 4. MRI T2 images showing a longitudinally extensive central hyperintensity extending from C1 to D5. Notice the mild cord enlargement. In general encephalitis/myelitis is an acute inflammatory process that affects brain or spinal cord tissue and is almost always accompanied by inflammation of the adjacent meninges. The disease is most commonly caused by viral infection. Encephalitis resulting from viral infection manifests as either acute viral encephalitis or postinfectious encephalomyelitis. Acute viral encephalitis is caused by direct viral infection of neural cells with associated perivascular inflammation and destruction of gray matter. Postinfectious encephalomyelitis follows infection with various viral or bacterial agents; the primary pathologic finding is demyelination of white matter. Postinfectious encephalitis/myelitis is an immunological disorders in which peripheral blood lymphocytes cross-react against myelin basic protein resulting in myelinolysis and inflammatory demyelination of the white matter. Breakdown of the blood brain barrier results in the formation of vasogenic edema that migrate along white matter tracts and is probably responsible for the MRI T2 hyperintensity observed in these disorders. Histologically, the acute lesions in Postinfectious encephalitis/myelitis are characterized by an extensive loss of myelin (perivenous cuffing with inflammatory cells, especially lymphocytes and macrophages, and loss of myelin). This may be in the form of a well-demarcated area of demyelination, although in the acute situation, the edges of the demyelinated lesions often are less well defined, and the demyelination and attendant cellular processes extend into the surrounding rim. Demyelinated fibers may be recognized by an axon devoid of a sheath, as seen histochemically, or immunohistochemically, or on electron microscopy by the presence of naked axons. In addition, thinly myelinated fibers may be seen within the lesion, suggesting partially demyelinated or remyelinated fibers. The presence of oligodendrocytes showing the re-expression of myelination proteins suggests the latter event is occurring in a least a significant number of these fibers. Vasogenic edema (due to breakdown of blood brain barrier) may be severe, and is seen as an expansion of the extracellular space, spreading apart both fibers and cells. Accompanying the myelin loss is a large infiltrate of foamy or debris- filled macrophages lying in sheets that appear to have replaced the normal neuropil. They also may be around the blood vessels, or infiltrating the more preserved areas of tissue as single cells. Depending on the age of the lesion, the macrophages may contain some or none of the myelin proteins described above, or may be LFB positive. The macrophages will stain for general markers such as KPI but depending on the patient's age, early
- 4. (MRP14) or late (27ElO) markers also may be present to help date lesions. The inflammatory infiltrate varies, but in most acute cases will be of some significance. Lymphocytes staining with the leukocyte common antigen comprise most cells, although polymorphonuclear leukocytes, eosinophils, plasma cells, and even mast cells have been found, together with less well-characterized monocytes. Although they may be present throughout the tissue, they are particularly prominent around the blood vessels, and at times may be so severe as to mimic a vasculitis. Both CD4 helper cells and CD8 suppressor cells may be found in the lesions. In the past, there have been suggestions that CD4 cells predominate in early lesions, with CD8 cells taking over at later stages, but this is variable, and a fixed pattern has not been defined. Many workers also have described the occurrence of gamma-delta lymphocytes in these lesions, and their association with acute phase reactant or stress proteins such as heat shock protein on oligodendrocytes has been well recognized. (MS) [72] Demyelination of the white matter is associated with breakdown of the blood brain barrier and the development of vasogenic edema. Vasogenic edema is the most common type of edema results from local disruption of the blood brain barrier. This leads to extravasation of protein-rich filtrate of plasma into the interstitial space, with subsequent accumulation of vascular fluid. This disruption results from loosening of the tight junctions between endothelial cells, and the neoformation of pinocytic vesicles. Once the barrier is breached, hydrostatic and osmotic forces work together to extravasate intravascular fluid. Once extravasated, fluid is retained outside the vasculature, mostly in the white matter of the brain, and within the bundles of myelinated axons of long tracts and commissural fibers. This is because axons run in parallel bundles of fibres with loose extracellular space (that offer low resistance and facilitates the extension of vasogenic edema along myelinated axons which are spreaded apart by the edema) as opposed to gray matter, which has high cell density and is enmeshed in an interwoven network of connecting fibres that offer high resistance to the formation and spread of edema. By definition, this type of edema is confined to the extracellular space. Vasogenic edema is responsible for the MRI T2 hyperintensity and MRI T1 hypointensity and The MRI T1 contrast enhancement frequently observed in these disorders. [72] Vasogenic edema fluid is retained outside the vasculature, mostly in the white matter of the brain, and within the bundles of myelinated axons of long tracts and commissural fibers. This is because axons run in parallel bundles of fibres with loose extracellular space (that offer low resistance and facilitates the extension of vasogenic edema along myelinated axons which are spreaded apart by the edema) as opposed to gray matter, which has high cell density and is enmeshed in an interwoven network of connecting fibres that offer high resistance to the formation and spread of edema. Vasogenic edema is probably responsible for the multisegmental MRI T2 hyperintensity that are commonly seen in postinfectious transverse myelitis that apparently spare gray matter (gray matter is commonly seen as the central dot sign which represents the gray matter squeezed by edema). In postinfectious transverse myelitis vasogenic edema travel up and down along white matter tracts resulting in the multisegmental involvement of the spinal cord that is characteristic of postinfectious transverse myelitis. [72] DIAGNOSIS: DIAGNOSIS: NEUROMYELITIS OPTICA (DEVIC'S DISEASE) DISCUSSION DISCUSSION: Neuromyelitis optica (NMO; also known as Devic’s syndrome or Devic’s disease) is an inflammatory disorder with a striking predilection for the optic nerves and spinal cord. Acute transverse myelitis is often its initial manifestation. The cardinal features of NMO (optic neuritis and myelitis) and tendency to recurrence led to its classification as a subtype of multiple sclerosis (MS), but it has several unique features. Herein, [I] describe the clinical, radiological, and pathological features of NMO, its pathogenesis, and its relationship to other forms of central nervous system demyelinating disease.
- 5. Clinical Features and Diagnostic Criteria Devic’s syndrome consists of one or more clinical episodes of optic neuritis in combination with myelitis. These clinical events also occur commonly in typical MS, however, in NMO they are usually more acute (sometimes fulminant) and severe; these characteristics may raise initial diagnostic suspicion of NMO. Paraclinical measures, such as magnetic resonance imaging (MRI) of the brain and spinal cord and cerebrospinal fluid (CSF) examination, also frequently reveal findings that differ from those in prototypic MS. In retrospective and small prospective series, most patients with NMO have no or few nonspecific white matter lesions on brain MR imaging. Spinal cord MR imaging also shows distinctive findings: a majority of patients have longitudinally extensive lesions extending over three or more vertebral segments. Furthermore, NMO patients frequently have a CSF pleocytosis of more than 50 leukocytes, with or without the presence of neutrophils. Three groups have proposed diagnostic criteria that employ some or all of these features. With the development of these criteria the following key findings have become accepted: 1.The interval between the initial events of ON and myelitis is quite variable (several years, in some instances); 2.Some patients experience unilateral rather than bilateral optic neuritis; and 3.The course may be monophasic or relapsing. Neuromyelitis optica may follow either a monophasic or relapsing course. [3] In monophasic NMO, patients experience either unilateral or bilateral ON and a single episode of myelitis, typically but not always, within a very short time of one another, but do not have further attacks. In contrast, patients with a relapsing course continue to have discrete exacerbations of ON and/or myelitis after they meet NMO diagnostic criteria. Acute transverse myelitis (ATM) Acute transverse myelitis (ATM) is an inflammatory demyelinating disorder that affects the spinal cord focally resulting in motor sensory and autonomic dysfunction. Establishing the diagnosis of ATM is not as difficult as determining the possible etiology. There is a difference in the perception of ATM seen in the West as compared to developing countries. In the West multiple sclerosis (MS) is the most common inflammatory disorder of the central nervous system. An attack of ATM may be the beginning of MS. However, this may not be the case in developing countries where MS is uncommon. Most often transverse myelitis is monophasic and at best represents a site-restricted form of acute disseminated encephalomyelitis (ADEM). Traditionally the combination of optic neuritis and ATM, occurring as a monophasic illness would have been called as neuromyelitis optica (NMO). Changing concepts in the definition of neuromyelitis optica and the discovery of a biomarker, neuromyelitis optica immunoglobulin (NMO_IgG), has changed the way relapsing autoimmune disorders are being perceived currently. A variety of idiopathic inflammatory disorders such as Japanese form of optic spinal MS, recurrent myelitis, and recurrent optic neuritis have been brought under the umbrella of neuromyelitis spectrum disorders because of the association with NMO-IgG. Complete transverse myelitis accompanied by longitudinally extensive transverse myelitis which is seronegative for this biomarker has also been reported from several countries including Japan, Australia, and India. Thus, ATM is a heterogeneous disorder with a varied clinical spectrum, etiology, and outcome. Acute transverse myelitis (ATM) is a pathogenetically heterogeneous inflammatory disorder of the spinal cord. An incidence of 1-4 per 100,000 has been reported in Western literature. [1] ATM has been reported as the major cause of noncompressive myelopathy. [2,3] A variety of disorders can cause ATM and includes infections, para and postinfections, and vascular, neoplastic, paraneoplastic, collagen vascular, and iatrogenic irregularities. [4] Until recently, it was believed that all relapsing idiopathic inflammatory diseases were multiple sclerosis
- 6. (MS). [4] With revisions in criteria for neuromyelitis optica (NMO), [5,6] the traditional concept of neuromyelitis optica being a monophasic disorder has been set aside. It is now accepted that it is characterized by severe attacks of optic neuritis (OPN) and myelitis which spares the brain, especially early in its course. Unlike MS there is a specific biomarker for neuromyelitis optica, neuromyelitis optica immunoglobulin G (NMO-IgG). This is an autoantibody present in the serum of patients with neuromyelitis optica which distinguishes neuromyelitis from other demyelinating disorders. [7] neuromyelitis optica-IgG binds to aquaporin-4 which regulates water homeostasis in the central nervous system (CNS). [8] It has also been detected in some patients with recurrent myelitis associated with longitudinally extensive spinal cord lesions (LESCLs), optic spinal MS (OSMS) seen in Japan (Asian form of multiple sclerosis), recurrent isolated optic neuritis, and optic neuritis or myelitis associated with certain autoimmune disorders. [9] Figure 5. Parainfectious transverse myelitis with longitudinally extensive hyperintense spinal cord lesions The transverse myelitis (TM) consortium [10] attempted to formulate a definition which would help to establish uniform diagnostic criteria and at the same time exclude other conditions that mimic this disorder. However, this definition does not help the clinician in deciding whether a given case has chances of converting to MS, whether it is a restricted from of acute disseminated encephalomyelitis (ADEM), or whether it belongs to the neuromyelitis optica spectrum of disorders. A clue to clinical diagnosis lies in the presentation: acute partial TM (APTM) or acute complete TM (ACTM). It also lies in its clinical course: monophasic or relapsing, and also in the clinical accompaniments such as optic neuritis. The nature and extent of lesions on the magnetic resonance imaging (MRI) of the spinal cord and the presence and distribution of brain lesions are important. Lastly, the seropositivity for aquaporin-4 antibody, otherwise called neuromyelitis optica-IgG antibody, is important, though in the current scenario it is not feasible to do it routinely especially in developing countries. Definitions It is important that the nosology of terms used in relation to TM is agreed upon and commonly used. Lack of uniformity creates diagnostic confusions and overlap between disease entities. The classical example would be the term Optic spinal multiple sclerosis . This term was loosely coined to describe MS with clinical attacks confined to the spinal cord and optic nerve in the earlier literature from. With the advent of MRI, Japanese authors have used this term to describe recurrent severe TM and optic neuritis which are accompanied by longitudinal spinal cord lesions on MRI. [11] This definition closely resembles the revised criteria for neuromyelitis optica. [4,5] In addition, the term spinal MS (SMS) has also been
- 7. used to describe recurrent TM. [12] Figure 6. A case with acute idiopathic transverse myelitis. Notice spinal cord swelling and the MRI T2 central hyperintensity and the central dot sign. Also notice the involvement of the complete cross section of the spinal cord. Acute complete transverse myelitis Acute complete transverse myelitis [13] may be defined as an idiopathic inflammatory disorder causing symmetric spinal cord dysfunction below a specific level of cord function. The ensuing disability may be moderate to severe. Acute monophasic TM has been described in the background of variety of infections and vaccinations [See table 1]. It may also be seen in the setting of ADEM, in nearly a quarter of patients. [14] Severe forms of ATM have been described with other autoimmune disorders such as systemic lupus erythemtosis (SLE), Sjfgren's syndrome (SS), primary antiphospholipid antibody syndrome, sarcoidosis, and various forms of vasculitis. [9] It may be the initial manifestation in as much as 23% of patients later diagnosed to have SLE. [15] Table1: Diseases Associated with transverse myelitis transverse myelopathy or radiculomyelitis Parainfectious (occurring at the time of and in association with an acute infection or an episode of infection). Viral: herpes simplex, herpes zoster, cytomegalovirus, Epstein-Barr virus, enteroviruses (poliomyelitis, Coxsackie virus, echovirus), human T-cell, leukemia virus, human immunodeficiency virus, influenza, rabies Bacterial: Pyogenic, Mycoplasma pneumoniae, Lyme borreliosis, syphilis, tuberculosis, Neuroschistosomiasis Postvaccinal (rabies, cowpox) Systemic autoimmune disease Systemic lupus erythematosis and other connective tissue disease Sjogren's syndrome Sarcoidosis Multiple Sclerosis
- 8. Paraneoplastic syndrome Vascular Thrombosis of spinal arteries Vasculitis secondary to heroin abuse Spinal arterio-venous malformation Antiphospholipid syndrome Radiation induced When ATM presents symmetrically with moderate to severe spinal cord dysfunction, the obvious implication is that chances of conversion to MS is remote. The conversion rate is less than 2% at five-year follow-up. [16] Rarity of ACTM in MS has also been highlighted in other studies. [17] Pediatric ATM is most often post infectious and may have a better outcome than adult patients. In addition, recurrence in myelitis and conversion to MS are rare. [18] Prognosis may be better for ATM occurring with ADEM than in isolated ATM. [19] Acute partial transverse myelitis In contrast to ACTM, APTM [20] may be defined as an asymmetrical or mild loss of spinal cord function. These patients may have patchy sensory impairment, mild to moderate weakness of asymmetric distribution, and occasional bladder dysfunction. Patients with APTM have greater chances of converting to MS. In one of the earlier studies on APTM, Ford et al , [21] have clearly shown that majority of patients presenting with asymmetric and patchy spinal cord dysfunction had converted to clinically definite MS (CDMS) within three years. Thirteen out of 15 patients (87%) who converted had abnormal brain MRI at onset of disease. When the initial brain MRI was normal, over a five-year follow-up period, approximately 20-30% converted to definite MS. [21] In patients presenting with APTM, detection of oligoclonal bands at onset or abnormal evoked potential studies were however not reliable predictors for conversion to CDMS. [22] Patients in this group are also not at high risk for developing neuromyelitis optica, [23] though some patients can experience mild relapse in spinal cord symptoms during the course of the illness. In conventional MS, also referred to as Western form of MS, spinal cord lesions are usually fewer than two vertebral segments and occupy less than one-half of a spinal cross-section, preferentially involving the peripheral white matter. [24] The MRI picture characteristic of idiopathic transverse myelitis 1. A centrally located multisegmental (3 to 8 spinal segments) MRI T2 hyperintensity that occupies more than two thirds of the cross-sectional area of the cord is characteristic of transverse myelitis. The MRI T2 hyperintensity commonly shows a slow regression with clinical improvement. The central spinal cord MRI T2 hyperintensity represents evenly distributed central cord edema. MRI T1 Hypointensity might be present in the same spinal segments that show T2 hyperintensity although to a lesser extent. The MRI T2 hyperintensity is central, bilateral, more or less symmetrical and multisegmental. 2. MRI T2 central isointensity, or dot (within and in the core of the MRI T2 hyperintensity) might be present and is believed to represent central gray matter squeezed by the uniform, evenly distributed edematous changes of the cord. (central dot sign). It might not be of any clinical significance. 3. Contrast enhancement is commonly focal or peripheral and maximal at or near the segmental
- 9. MRI T2 hyperintensity. In idiopathic transverse myelitis enhancement is peripheral to the centrally located area of high T2 signal intensity rather than in the very same area. The prevalence of cord enhancement is significantly higher in patients with cord expansion. 4. Spinal cord expansion might or might not be present and when present is usually multisegmental and better appreciated on the sagittal MRI T1 images. Spinal cord expansion tapers smoothly to the normal cord, and is of lesser extent than the high T2 signal abnormality. 5. Multiple sclerosis plaques (and subsequent T2 hyperintensity) are located peripherally, are less than 2 vertebral segments in length, and occupies less than half the cross-sectional area of the cord. In contrast to transverse myelitis, enhancement in MS occurs in the same location of high-signal-intensity lesions seen on T2-weighted images. Table 2. Differences between idiopathic transverse myelitis and spinal multiple sclerosis Number T2 of Disease entity Contrast element Pathology hyperintensity segments involved Idiopathic Central, 4-8 In transverse myelitis Nonspecific necrosis that transverse myelitis multisegmental enhancement is peripheral affects gray and white to the centrally located area matter indiscriminately of high T2 signal intensity and destroys axons and cell rather than in the very same bodies as well as myelin. area. Spinal multiple Peripheral 1-2 In contrast to transverse White matter sclerosis myelitis, enhancement in demyelination only. MS occurs in the same location of high-signal- intensity lesions seen on T2- weighted images. Neuromyelitis optica Neuromyelitis optica diagnostic criteria have been revised twice in recent times. [5,6] Neuromyelitis optica may be monophasic or recurrent. The most recent criteria require acute optic neuritis and myelitis as absolute requirements. Alongside, two of the following supportive criteria are required: contiguous spinal cord MRI lesion extending over three vertebral segments, brain MRI not meeting diagnostic criteria for MS and Neuromyelitis optica-IgG seropositive status. Evolution of criteria for diagnosis of neuromyelitis optica The standard definition of Neuromyelitis optica (Devic's disease) was one of a monophasic disorder involving spinal cord and both the optic nerves, without involvement of the rest of the neuraxis. Though it was accepted that the underlying pathology was inflammatory, opinion was divided as to whether it represented a distinct disease, a variant of MS, or a postinfective restricted form of ADEM. [25,26] In 1999 Wingerchuck et al , [5] put forward the first of their revised criteria for Neuromyelitis optica. Three absolute requirements were optic neuritis, acute myelitis, and no symptoms implicating other CNS regions. Fulfillment of at least one of three major supportive criteria was required: 1) normal brain MRI at disease onset or MRI not fulfilling MS imaging criteria; 2) spinal cord MRI showing a lesion extending over >3 vertebral segments; and 3) cerebrospinal fluid (CSF) revealing >50 WBC/mm 3 or >5 neutrophils/mm. [3] Alternatively, fulfilling two of three minor supportive criteria (bilateral optic neuritis, severe residual visual loss, or severe fixed postattack weakness) would suffice. In 2006, the Neuromyelitis optica diagnostic criteria [5] were revised to include patients who had features
- 10. of Neuromyelitis optica but had additional sites of neurological dysfunction or brain MRI lesions not consistent with MS [Table 3]. The most notable features of this revised criteria were obviously the inclusion of newly detected biomarker - Neuromyelitis optica-IgG. Nonspecific brain lesions can develop in 60% of patients which immunohistochemically resemble spinal cord lesions of Neuromyelitis optica. [27] MRI lesions typical of MS can develop in 10% of patients who otherwise fulfill criteria for Neuromyelitis optica. In another 10%, white matter lesions can develop in aquaporin-rich periependymal regions such as hypothalamus and the periaqueductal brainstem. [28] Earlier it was thought that brain lesions in Neuromyelitis optica were asymptomatic, but now it is accepted that some lesions may be symptomatic - for example, the nonautoimmune endocrinopathies reported in association with Neuromyelitis optica. More than 90% of patients fulfilling the criteria have a relapsing course rather than monophasic and have recurrent optic neuritis and myelitis. [29] Table 3. Longitudinally extensive transverse myelitis Spinal cord lesion length has been emphasized as an important distinguishing factor between Neuromyelitis optica and MS. Longitudinally extensive transverse myelitis (LETM) or LESCLs or long cord lesions (LCL) refers to idiopathic spinal cord inflammatory lesions extending more than three vertebral segments on the MRI. Neuromyelitis optica-IgG seropositivity appears to be much more likely in patients presenting with lesions extending over multiple spinal levels compared with patients with small single-level cord lesions. [30] In conventional MS reported from the West, 3-12.5% of patients have been reported to have LETM. [24,31] Longitudinal cord lesions are seen in 25% of Japanese patients with conventional MS. [32] In the Japanese form of Optic spinal multiple sclerosis, the incidence is much higher, 59%. However, it is worth noting that the Japanese definition of Optic spinal multiple sclerosis closely resembles that of Neuromyelitis optica as mentioned earlier. Clinical features - Neuromyelitis optica versus multiple sclerosis Relapsing Neuromyelitis optica has a 5:1 preponderance in women, though the monophasic variant is equally distributed among sexes. The mean onset of symptoms is later in Neuromyelitis optica, most often in the third decade rather than the second (in MS). The severity of visual loss and the incomplete recovery is a hallmark of Neuromyelitis optica. Patients are conscious of their visual loss. In MS, very often pallor of the optic disc detected on clinical examination or a delayed visual evoked response draws attention to the underlying optic neuritis. Complete transverse myelitis and the incomplete recovery are other clinical features that should alert the physician. The occurrence of hiccoughs or respiratory failure during the course of myelitis should raise suspicion for Neuromyelitis optica. [29] The course of the disease is distinctly different from MS. Relapse occurs within one year in 60% and within three years in 90%. [5] Within five years of disease onset, more than 50% of patients with relapsing remitting Neuromyelitis optica are blind in one or both eyes or require ambulatory support (Neuromyelitis optica spectrum disorders). The calculated five-year survival rate for Neuromyelitis optica is 68%, all deaths are related to neurogenic respiratory failure. [33] In comparison the course in relapsing remitting MS is different. Attacks are mild with good recovery and permanent disability generally builds up over the years during the secondary progressive phase of the disease. A secondary progressive phase is rare in Neuromyelitis optica. [34]
- 11. The immunopathology of neuromyelitis optica and the neuromyelitis optica-IgG Neuromyelitis optica lesions are characterized by necrotizing lesions involving both gray and white matter of the spinal cord extending across many segments and resulting in cavitations. [35] Inflammatory infiltrates of active lesions in Neuromyelitis optica have eosinophils and neutrophils, a feature unique to Neuromyelitis optica and not seen in MS. Penetrating spinal vessels are thickened and hyalinized. [36] Immunoglobulin and compliment components are deposited in a characteristic vasculocentric rim and rosette pattern in active lesions. This pattern corresponds to normal expression of aquaporin-4 in the end feet of astrocytes. [37,38] In contrast to MS lesions, in which aquaporin-4 immunoreactivity is increased, aquaporin-4 is absent in Neuromyelitis optica lesions. [37,39] The Neuromyelitis optica-IgG, an autoantibody reported by Lennon and colleagues, was found to be 73% sensitive and 91% specific for 124 clinically defined Neuromyelitis optica patients studied prospectively. [7] Neuromyelitis optica-IgG binds to aquaporin-4 which is the main channel that regulates water homeostasis in the CNS. Subsequently, data from other European countries confirmed the sensitivity and specificity of Neuromyelitis optica-IgG in differentiating Neuromyelitis optica from MS. The authors have however admitted that 10-25% of patients with clinically diagnosed Neuromyelitis optica were seronegative for Neuromyelitis optica-IgG. Neuromyelitis optica-IgG may also have a predictive value. Weinshenker et al , [40] evaluated 29 patients with a first attack of TM with an MRI lesion spanning three or more vertebral segments. Of 23 patients followed for one year, 9 were seropositive for the Neuromyelitis optica-IgG autoantibody. Within one year, five of nine patients had a second event, involving recurrent TM in four patients and optic neuritis in one patient. After 1-7 years of follow up, none of the 14 patients who were seronegative for the Neuromyelitis optica-IgG autoantibody had a relapse of myelitis or optic neuritis. Expanding spectrum of neuromyelitis optica A variety of allied disorders is grouped under the spectrum of Neuromyelitis optica, based on the detection of Neuromyelitis optica-IgG in the serum of affected patients reported from select centers. This includes Asian Optic spinal multiple sclerosis (reported from Japan), recurrent myelitis associated with LESCLs, recurrent isolated optic neuritis, and optic neuritis or myelitis in the context of certain organ- specific and nonorgan-specific autoimmune disorders such as SLE and SS. [9] Optic spinal multiple sclerosis The detection of Neuromyelitis optica-IgG in select group of patients with Optic spinal multiple sclerosis from Japan, led to the conclusion that Japanese patients with Optic spinal multiple sclerosis may actually have Neuromyelitis optica. In 12 out of 19 patients (65%) with Optic spinal multiple sclerosis and in 2 out of 13 patients with CMS, Neuromyelitis optica-IgG was positive. [41] Features of Japanese Optic spinal multiple sclerosis include: (1) older age at onset, (2) female preponderance, (3) frequent relapses, (4) greater disability due to severe optic nerve and spinal cord damage, (5) fewer brain lesions detected by MRI, (6) LESCLs extending over many vertebral segments on spinal cord MRI, (7) marked pleocytosis and neutrophilia in CSF, and (8) absence of oligoclonal bands in CSF. [11] These observations are almost identical to the criteria proposed for Neuromyelitis optica and are at variance with definitions used in other studies and may explain the high degree of seropositivity in this Japanese series. Out of the original cases studied by Nakashima et al , [41] two of the cases labeled as CMS and seropositive for Neuromyelitis optica-IgG on review were found to have LETM on MRI. This exemplifies the need for uniform diagnostic criteria and the fact that perhaps length of spinal cord lesions correlated best with seropositivity in Japanese Optic spinal multiple sclerosis. In a more recent study, Matsuoka and colleagues [42] have studied consecutive sera from 113 patients with MS which included Optic spinal multiple sclerosis (48) and CMS (54). Interestingly only 15 (27.1%) of Optic spinal multiple sclerosis showed seropositivity. When the MRI data were incorporated, it was found that 9 out of 48 Optic spinal multiple sclerosis patients had LETM and 5 of them were seropositive for Neuromyelitis optica-IgG. Compared to the earlier Japanese study, Neuromyelitis optica-IgG seropositivity was much lower.
- 12. Kira et al , [7] commented on the obvious differences in seropositivity among Japanese patients. The series of Takahashi et al , [43] (20/22, 91% in Neuromyelitis optica patients), Tanaka et al , [44] (60% in Optic spinal multiple sclerosis patients with LESCLs), and Matsuoka et al , [45] (11/31, 35% in Optic spinal multiple sclerosis patients with LESCL) had varying results for the Neuromyelitis optica-IgG assay. On analysis of these studies, [7] it was found that there was no homogeneity in patient selection, those having LETM were lumped with patients having shorter cord lesions. In addition, some of the studies included only female [43] or predominantly female patients and were selected from an existing database rather than consecutive cases. Matsuoka et al , [42] who had the lowest seropositivity, studied consecutive patients and included both sexes. At one study center in Japan [7] the sensitivity of Neuromyelitis optica-IgG detection assay was increased to 100% without any improvement noted in the detection rates. This eliminated the variation in seropositivity due to poor assay sensitivity. Recurrent myelitis Relapsing myelitis has been recognized as a condition distinct from MS. [45,46] Recurrent CTM associated with longitudinal spinal cord lesions was more likely to be Neuromyelitis optica-IgG seropositive in Western case series. [9] As mentioned earlier, Neuromyelitis optica-IgG seropositivty after the first attack of ACTM predicts a relapsing course in more than 50% of patients within three years of the first attack. Myelitis associated with organ-specific and nonorgan-specific autoimmune disease Weinshenker et al , [47] observed Neuromyelitis optica-IgG seropositivty in some patients with SLE and SS who had Neuromyelitis optica or Neuromyelitis optica spectrum disorders. In contrast, SLE and SS associated with systemic autoimmune disease and uncomplicated by Neuromyelitis optica were seronegative for the biomarker. Pittock et al , [48] from the same group found a higher frequency of nonorgan-specific autoantibodies in Neuromyelitis optica-IgG-positive patients than in Neuromyelitis optica-IgG-negative patients. Nonorgan-specific autoimmunity was however seen equally in Neuromyelitis optica seropositive and negative cases reported from a Japanese study of Optic spinal multiple sclerosis. [41] Figure 7. A 34-year-old male who presented with acute partial transverse myelitis had MRI cervical spine showing single linear enhancing lesion extending less than three vertebral segments. Within eight months he developed right optic neuritis and was labeled as multiple sclerosis
- 13. Figure 8. A 37-year-old female who had recurrent episodes of optic neuritis and myelitis, had MRI cervical spine T2W images showing longitudinally extensive myelitis extending into the cervicomedullary region. She was Neuromyelitis optica-IgG seropositive Figure 9. A 48-year-old female who had episodes of recurrent acute partial transverse myelitis and optic neuritis with initial improvement between relapses. She later developed blindness in right eye and severe quadriparesis. (A) T2-W MRI of spinal cord showing linear coalescing cervical spinal cord lesion extending more than three vertebral segments; (B, C) Axial FLAIR images of the brain showing periventricular and subcortical discrete demyelinating lesions. She was Neuromyelitis optica-IgG positive Pandit and colleagues analyzed Neuromyelitis optica-IgG status in 78 consecutive cases obtained from their demyelination registry. [56,57] In this study 63/78 (81%) patients belonged to the Neuromyelitis optica spectrum. Longitudinally extensive transverse myelitis was seen in 51% and included all cases of Neuromyelitis optica, ATM, and recurrent ATM. Neuromyelitis optica-IgG was positive in three female patients (3.8%), one each of Neuromyelitis optica, Optic spinal multiple sclerosis, and recurrent ATM. Seropositive patients were all women and had late onset disease. Both patients with optic neuritis had severe visual impairment and all three were wheel chair bound within five years of disease onset. Seronegative patients (36) had M/F ratio: 2:1 and had mild visual impairment. Assistance for mobility was required by 30% (12). The remaining patients recovered well between attacks and remained ambulant 4-7 years after disease onset.
- 14. An earlier clinical study by Pradhan et al , [58] showed a similar outcome. They reported six patients, three male and female patients, each who had recurrent CTM and optic neuritis. Brain MRI was normal, but in all, spinal cord MRI showed LETM extending 6-9 segments. These patients on follow up, 2-10 years later, were independently ambulant except for one patient who required a cane. It is clear that seronegative cases far exceed Neuromyelitis optica-IgG-positive patients. Additionally, the clinical behavior of patients in both studies quoted above distinguished them as separate entities. They had milder spinal cord and visual disturbances in spite of LETM, during a lengthy follow-up period. There are only few studies reporting low seropositivity [59,60] among Caucasian patient populations. Given that the proponents of the concept of Neuromyelitis optica spectrum disorders [9] admitted that 25% of patients tested by them were negative for Neuromyelitis optica-IgG. There have been no studies analyzing these seronegative patients especially from the West. From Japan, Matsuoko and colleagues [42] in their landmark paper highlighted that a significant number of their Optic spinal multiple sclerosis patients were seronegative (65%). Optic neuritis in the seronegative cases was less severe. They had LETM which differed from seropositive patients. MRI lesions in the seronegative group appeared throughout cervical to thoracic cord as compared to seronegative patients who had lesions in the upper or midthoracic cord. On axial sections, seronegative patients had a holocord appearance compared to central gray matter involvement in seropositive patients. Other Japanese studies have also noted a lesser frequency of relapses and milder visual disturbances in their seronegative patients. [41,44] These studies sharply highlight the growing view that there is heterogeneity in the mechanisms producing longitudinal inflammatory lesions, some of which may be independent of aquoporin-4 autoimmunity. The need to distinguish multiple sclerosis from neuromyelitis optica The importance of separating Neuromyelitis optica from MS is twofold. Firstly, Neuromyelitis optica has a worse outcome than MS, with frequent and early relapses. Within five years of onset, 50% of patients are blind in both eyes and cannot walk unassisted, and 20% die of respiratory failure due to cervical myelitis. Secondly, Neuromyelitis optica responds to immunosuppressive therapy with agents such as azathioprine and rituximab, [61,62] whereas the currently promoted treatment of MS includes immune- modulating agents such as interferon. Optic spinal form of MS cases in Japan which were seronegative for Neuromyelitis optica-IgG responded well to interferon even when there was concomitant LETM. [42] Optic spinal multiple sclerosis sometimes most likely to be closer to conventional MS than Neuromyelitis optica and therefore should be given the benefit of treatment with disease modifying agents such as beta interferon. Conclusion A relatively familiar condition, ATM has become transformed with recent developments, especially the advent of the MRI and the discovery of biomarker Neuromyelitis optica-IgG. It is the clinician's responsibility to label ATM according to internationally established criteria, starting with a clinical description of partial or complete TM. Imaging studies should be done in the acute phase of the illness so as to accurately measure the length of spinal cord lesions and should include the brain as well. Reasonable investigations should be done to establish the idiopathic nature of ATM. A long-term follow-up study helps to reasonably ascertain conversion to MS or an Neuromyelitis optica phenotype disorder. Prior to the detection of Neuromyelitis optica-IgG antibody, opinions differed as to whether MS and Neuromyelitis optica were the same or distinct disorders. However, the observations that Neuromyelitis optica-IgG is not found in all cases of Neuromyelitis optica or Neuromyelitis optica spectrum disorders and that about 10% of classical MS patients also carry the antibody, erodes its credibility. The question that needs to be answered once again is whether, based on this biomarker, we should reclassify CNS demyelinating disorders into MS and autoimmune aquaporinopathies. Is Neuromyelitis optica-IgG responsible for disease causation or is it simply an epiphenomenon?
- 15. SUMMARY SUMMARY Clinical picture or neuromyelitis optica The cardinal clinical features of the disorder are transverse myelitis, which is often longitudinally extensive, and optic neuritis. These two index events can occur simultaneously, in rapid succession, or they can be separated by many years. The optic neuritis can be unilateral or bilateral. Some patients have repeated episodes of optic neuritis before myelitis occurs and vice versa (the nomenclature of the disease at this stage is relapsing myelitis or relapsing optic neuritis). Most of those affected (>80%) go on to have repeated relapses (relapsing NMO) though a minority may have only the index events (monophasic disease). Relapses are generally more disabling than those in patients with typical MS. Whereas in MS disability develops largely in the progressive phase of the disease, in neuromyelitis optica disability is acquired as a consequence of relapses; progressive disability without relapses is rare in our experience. In white populations most patients presenting with optic neuritis and myelitis are likely to have MS rather than neuromyelitis optica. Features which may help distinguish the disorder from MS clinically are the more severe myelitis, optic neuritis with poor recovery, and no involvement of other parts of the neuraxis. However the disease spectrum may be wider than currently accepted. A variety of diagnostic criteria for the disorder have been formulated and are summarised elsewhere. [68] None is perfect and it is likely that they will be revised in the light of emerging clinical and laboratory data. In general we would consider the diagnosis in the presence of: Longitudinally extensive myelitis (usually more than three vertebral segments) Optic neuritis Normal brain MRI, or if abnormal, atypical for MS-see below. Clinical, radiological, and immunopathological studies suggest neuromyelitis optica is distinct from MS The prognosis also differs from MS. Early reports suggested a five year survival of 68%, death often resulting from severe spinal cord disease and respiratory compromise. [67] More recent series suggest a better outcome, possibly reflecting better case ascertainment or treatment. However there is no doubt that disability is acquired earlier in neuromyelitis optica than MS; this and a mean relapse rate of around two per year make early diagnosis and therapy imperative. Neuroimaging or neuromyelitis optica The most characteristic radiological feature is a longitudinally extensive cord lesion, extending often over three or more spinal segments and expanding the cord (fig 1). This is usually hypointense on T1 and hyperintense on T2 MRI. Lesions are centrally situated within the cord and patchy contrast enhancement is often seen. Occasionally lesions can be identified in the optic nerves. Though classically NMO has been defined by lack of brain lesions or symptoms, it has increasingly been noted that up 60% of patients with otherwise typical relapsing NMO (many with positive NMO-IgG) can have lesions on brain MRI. These range from extension of high cervical cord lesions into the brain stem, diencephalic, brainstem or cerebral lesions "atypical" for MS and in minority MS- like lesions. However all these patients have long cord lesions which seems to be a specific feature, distinguishing these cases from MS. [71] Laboratory investigations of neuromyelitis optica
- 16. Cerebrospinal fluid acutely may reveal a prominent pleocytosis of either lymphocytes or neutrophils and raised protein. In contrast to MS, there are usually no oligoclonal bands (in over 80%). Lennon and co- workers at the Mayo clinic recently reported the discovery of NMO- IgG, which may be the first "disease specific" antibody in CNS demyelinating disease. [65]The antibody, identified initially from Western blots in patients screened for possible paraneoplastic antibodies, is reported to have a sensitivity of 73% and a specificity 91% for neuromyelitis optica and was also positive in a significant proportion of patients deemed to be at high risk of neuromyelitis optica (that is, patients with recurrent optic neuritis or myelitis). Despite the apparent association of NMO- IgG with neuromyelitis optica, independent confirmation of this finding and indeed evidence of pathogenesis is still awaited. The Mayo group has also recently reported that the target antigen for NMO-IgG appears to be the aquaporin-4 water channel, located in astrocytic foot processes at the blood-brain barrier. [64] To date, testing for NMO-IgG is only available through the Mayo clinic (Rochester, USA; at a cost of $500/sample). If the role of the antibody is confirmed and the assay becomes readily available, the hope is clearly that the relation between neuromyelitis optica and "neuromyelitis optica spectrum" disorders (see below) will be clarified, and predictive testing for neuromyelitis optica in patients with isolated myelitis or severe optic neuritis may be possible. A range of positive auto-antibodies (including ds-DNA) have been reported in up to 40% of patients. [66] This can give rise to diagnostic difficulties. We would consider these to be epiphenomena occurring in the context of disordered humoral immunity. However there are reports of patients with unambiguous systemic lupus erythematosus, Sjögren's syndrome, and mixed connective tissue disease who have developed neuromyelitis optica. Whether they have two separate autoimmune diseases, or neuromyelitis optica is a consequence of the primary disorder, is impossible to determine clinically. Testing for NMO- IgG and pathological examination in such cases may be able to clarify this. Pathology of of neuromyelitis optica Extensive necrosis, demyelination, and often cavitation across multiple spinal cord segments, involving grey and white matter with perivascular infiltrates, prominent macrophages, eosinophils, and vascular hyalinisation is typical. Deposition of complement in a ring pattern on the outer surface of blood vessels and in a rosette perivascular pattern has been elegantly demonstrated. Prominent perivascular IgG reactivity and IgM deposition in a rosette pattern implicate these as sites of immune mediated damage. [70] Management of of neuromyelitis optica The rarity of the condition inevitably limits the evidence for therapeutic interventions. As in most immune mediated disorders management consists of treatment of relapses, therapy for the underlying disease, symptom control, and rehabilitation. For the general neurologist managing a patient with neuromyelitis optica the approach to relapses and indeed underlying disease therapy is perhaps most comparable to that of the more common antibody mediated disorder myasthenia gravis. Management of relapses High dose corticosteroids and supportive care remain the mainstays of management of relapse. In view of the severity of relapses and likely need for maintenance treatment our policy is to follow a course of intravenous methylprednisolone (1 gram daily for 3-5 days) with a gradual taper of oral prednisolone over several months, from an initial dose of 1 mg/kg/ day. An alternate day maintenance dose of the order of 10-20 mg prednisolone is generally our target in patients with relapsing disease. A minority of patients fail to respond to adequate steroid therapy or relapse rapidly and in such cases there is a role for therapeutic plasma exchange. We consider it early-within weeks of symptom onset. In the North American randomised trial of plasma exchange in severe demyelinating events, patients with neuromyelitis optica were overrepresented among the responders, with a 60% response rate (versus 6% overall for sham exchange). In this study plasma exchange was undertaken within three months of onset of relapse.
- 17. Prevention of relapses Most patients follow a relapsing course, often acquiring substantial disability within two or three relapses. Immunosuppression appears to reduce the relapse rate. The first report of successful treatment was a series of seven patients treated with prednisolone (tailing dose as above) and azathioprine (at 2.5-3 mg/kg). In the author experience, relapse rates were reduced by over 80% in patients established on immunosuppressive therapy (most frequently azathioprine). This has therefore remained the author's initial therapy option in patients with relapsing, but reasonably stable, disease. For patients intolerant of azathioprine, mycophenylate mofetil is a reasonable alternative (but without any evidence base), with the possible advantage of more rapid onset of action. Practical points 1. Although neuromyelitis optica is uncommon it is a rapidly disabling yet treatable disorder. 2. Early recognition and diagnosis followed by prompt, carefully supervised immunosuppressive treatment in relapsing patients is paramount. A blind, quadriparetic, ventilator dependent person, from a treatable disorder, is a tragedy that one should strive to avoid at all costs. 3. Management appears distinct from that of MS and should probably be in the hands of clinicians with an interest in demyelinating disease. In patients who have "breakthrough" disease on azathioprine, or who present with frequent severe relapses, more aggressive immunosuppression may be necessary. Recent small case series have reported on the successful use of rituximab (a B cell depleting monoclonal antibody) and mitoxantrone. The author have used both of these agents in small numbers of patients without complications. Interferon beta, the mainstay of treatment in relapsing MS, does not appear to be effective. Symptoms control and rehabilitation Pain, stiffness, bladder, and bowel symptoms need to be tackled. Tonic spasms seem to be much commoner than in MS and in our experience usually respond to carbamazepine. Rehabilitation, physiotherapy, mobility, and visual aids are often needed. Some patients with high cervical cord lesions will need long term home ventilatory support. There is a UK based self-help group (telephone +44 (0) 151529 6100) and a patient friendly website (http://www.thewaltoncentre.co.uk/patients/ Neuromyelitis_Optica.html) THE WIDENING SPECTRUM OF NEUROMYELITIS OPTICA There may be a number of related disorders, a full discussion of which is beyond the scope of this review. Patients with idiopathic relapsing myelitis, Asian "optico-spinal" MS, and chronic relapsing inflammatory optic neuropathy may form part of the "neuromyelitis optica spectrum". We have seen several patients presenting with severe myelitis without visual symptoms but delayed visual evoked responses, some of whom have subsequently developed optic neuritis. It seems likely that such patients have neuromyelitis optica although they fall outside current criteria for the disorder. Traditionally patients with clinical or radiological findings outside the optic nerves and spinal cord have also been excluded from the diagnosis of neuromyelitis optica. There are however recent reports of patients with clinically typical neuromyelitis optica who have developed brain lesions on MRI. Interestingly these patients have been shown to have positive NMO-IgG, and histopathology (of the brain lesions) similar to classical neuromyelitis optica, widening the spectrum still further. More studies with a validated disease marker in the near future should clarify these relationships. Genetic links between neuromyelitis optica and MS have been hypothesised and will perhaps open a window to the better understanding of both conditions. [71,72] Addendum
- 18. A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and remains available till Friday.) To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com". To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file. Click here to download the short case version of this case record in PDF format REFERENCES References 1. Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: Incidence and etiologic considerations. Neurology 1981;31:966-71. 2. Prabhakar S, Syal P, Singh P, Lal V, Khandelwal N, Das CP. Non-compressive myelopathy: Clinical and radiological study. Neurol India 1999;47:294-9. 3. Chaurasia RN, Verma A, Joshi D, Misra S. Etiological spectrum of non-traumatic myelopathies: Experience from a tertiary care centre. J Assoc Physics India 2006;54:445-8. 4. Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA. Transverse myelitis: Pathogenesis, diagnosis and treatment. Front Biosci 2004;9:1483-99. 5. Wingerchuck DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:17-38. 6. Wingerchuck DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485-9. 7. Lennon VA, Wingerchuck DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum antibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004;364:2106-12. 8. Amiry-Mogoddam M, Otterson OP. The molecular basis of water transport in the brain. Nat Rev Neurosci 2003;4:991-1001. 9. WingerchuckDM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of Neuromyelitis optica. Lancet Neurol 2007;6:805-15. 10. Transverse Myelitis consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499-505. 11. Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol 2003;2:117-27. 12. Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI
- 19. features of Japanese patients with multiple sclerosis positive for NMO- IgG. J Neurol Neurosurgery Psychiatry 2006;77:1073-5. 13. Scott TF, Kassab SL, Singh S. Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: Transition rate to clinically definite multiple sclerosis. Mult Scler 2005;11:373-7. 14. Bennetto L, Scolding N. Inflammatory/ Postinfectious encephalomyelitis. J Neurol Neurosurg Psychiatry 2004;75:22-8. 15. Espinosa G, Mendizabal A, M?nguez S, Ramo-Tello C, Capellades J, Oliv? A, et al. Transverse myelitis affecting more than 4 spinal segments associated with systemic lupus erythematosus: Clinical, immunological, and radiological characteristics of 22 patients. Semin Arthritis Rheum 2008 (E pub ahead of print). 16. Scott TF, Bhagavatula K, Snyder PJ, Chieffe C. Transverse myelitis comparison with spinal cord presentations of multiple sclerosis. Neurology 1998;50:429-33. 17. Simnad VI, Pisani DE, Rose JW. Multiple sclerosis presenting as transverse myelopathy: Clinical and MRI features. Neurology 1997;48:65-7 18. Defresne P, Hollenberg H, Husson B, Tabarki B, Landrieu P, Huault G, et al. Acute transverse myelitis in children: Clinical course and prognostic factors. J Child Neurol 2003;18:401-6. 19. Yiu EM, Kornberg AJ, Ryan MM, Coleman LT, Mackay MT. Acute transverse myelitis and acute disseminated encephalomyelitis in childhood: Spectrum or separate entities? J Child Neurol 2009;24:287- 96. 20. Ford B, Tampieri D, Francis G. Long-term follow-up of acute partial transverse myelopathy. Neurology 1992;42:250-2. 21. Scott TF, Kassab SL, Singh S. Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: Transition rate to clinically definite multiple sclerosis. Mult Scler 2005;11:373-7. 22. Bashir K, Whitaker JN. Importance of para clinical and CSF studies in the diagnosis of MS in patients presenting with partial cervical transverse myelopathy and negative cranial MRI. Mult Scler 2000;6:312-6. 23. Scott TF, Kassab SL, Pittock JS. Neuromyelitis optica IgG status in acute partial transverse myelitis. Arch Neurol 2006;63:1398-400 . 24. Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 1995;95:725-32. 25. Cree BA, Goodin DS, Hauser SL. Neuromyelitis optica. Semin Neurol 2002;22:105-22. 26. de Seze J. Neuromyelitis optica. Arch Neurol 2003;60:1336-8. 27. Pittock SJ, Lennon VA, Krecke K, Wingerchuck DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;6:390-6. 28. Pittock JS, Weinshenker BG, Lucchinetti CF, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964-8. 29. Wingerchuck DM. Diagnosis and treatment of neuromyelitis optica. Neurologist 2007;13:2-11. 30. Scott TF, Kassab S, Pittock SJ. Neuromyelitis optica antibodies and acute partial transverse myelitis. Arch Neurol 2006;63:1398-400.
- 20. 31. Bot JC, Barkoff F, Polman CH, Lycklama à Nijeholt GJ, de Groot V, et al. Spinal cord abnormalities in recently diagnosed MS patients: Added value of spinal MRI examination. Neurology 2004;62:226-33. 32. Kira J. Neuromyelitis Optica and Asian Phenotype of Multiple Sclerosis. Ann NY Acad Sci 2008;1142:58-71. 33. Wingerchuck DM, Weinshenker BG. Neuromyelitis optica: Clinical predictors of a relapsing course and survival. Neurology 2003:60:848-53. 34. Wingerchuck DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 2007;68:603-5. 35. Mandler RN, Davis LE, Jeffrey DR, Kornfeld M. Devic's neuromyelitis: A clinicopathological study of 8 patients. Neurology 1993;34:162-8. 36. Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450-61. 37. Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern specific loss of aquaporin 4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007;130:1194-205. 38. Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, et al. Loss of aquaporin 4 in lesions in neuromyelitis optica: Distinction from multiple sclerosis. Brain 2007;130:1224-34. 39. Sinclair C, Kik J, Herron B, Fitzgerald U, McQuaid S. Absence of aquaporin 4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal appearing white matter. Acta Neuropathol 2007;113:187-94. 40. Weinshenker BG, Wingerchuk DM, Vukusic S, Linbo L, Pittock SJ, Lucchinetti CF, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 2006;59:566-9. 41. Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO- IgG. J Neurol Neurosurgery Psychiatry 2006;77:1073-5. 42. Matsuoka T, Matsushita T, Kawano T, Osoegawa M, Ochi H, Ishizu T, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain 2007;130:1206- 23. 43. Takahashi T, Kazuo F, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Anti-aquaporin-4 antibody is involved in the patho-genesis of NMO: A study on antibody titre. Brain 2007;130:1235-43. 44. Tanaka K, Tani T, Tanaka M, Saida T, Idezuka J, Yamazaki M, et al. Anti-aquaporin 4 antibody in selected Japanese multiple sclerosis patients with longspinal cord lesions. Mult Scler 2007;13:850-5. 45. Tippett DS, Fishman PS, Panitch HS. Relapsing transverse myelitis. Neurology 1991;41:703-17. 46. Pandit L, Rao SN. Recurrent Myelitis. J Neurol Neurosurgery Psychiatry 1996;60:336-8. 47. Weinshenker B, De Seze J, Vermersch P. The relationship between neuromyelitis optica and systemic autoimmune disease. Neurology 2006;66:380. 48. Pittock SJ, Lennon VA, Wingerchuk DM. The prevalence of non-organ-specific autoantibodies and NMO-IgG in neuromyelitis optica (NMO) and related disorders. Neurology 2006;66:307.
- 21. 49. Dastur DK, Singhal BS. Two unusual neuropathologically proven cases of multiple sclerosis from Bombay. J Neurol Sci 1973;20:397-414. 50. Chopra JS, Radhakrishnan K, Sawhney BB, Pal SR, Banerjee AK. Multiple sclerosis in Northwest India. Acta Neurol Scand 1980;62:312-21. 51. Gangopadhyay G, Das SK, Sarda P, Saha SP, Gangopadhyay PK, Roy TN, et al. Clinical profile of multiple sclerosis in Bengal. Neurol India 199;47:18-21. 52. Wasay M, Khatri IA, Khealani B, Sheerani M. MS in Asian countries. Int MS J 2006;13:58-65. 53. Jacob A, Boggild M. Neuromyelitis optica. Ann Indian Acad Neurol 2007;10:231-90. 54. Bansil S, Singhal BS, Ahuja GK. Comparison between multiple sclerosis in India and the United States: A case-control study. Neurology 1996;46:385-7. 55. Pandit L, Shetty R, Bhat IG, Misri Z, Hegde S. Spectrum of MS and other demyelinating CNS disorders in the background of revised criteria. Ann Ind Acad Neurol 2007;10:44-5. 56. Pandit L. Neuromyelitis optica antibody (NMO-IgG) status in Indian patients with multiple sclerosis and allied demyelinating disorders. Neurol Asia 2008;13:175-8. 57. Pandit L. NMO-IgG in Indian patients with Multiple sclerosis and allied idiopathic inflammatory demyelinating disorders. Mult Scler 2009;15:131. 58. Pradhan S, Mishra VN. A central demyelinating disease with atypical features. Mult Scler 2004;10:308-15. 59. Wu JS, Matsushita T, Carroll WM, Kira J, Mastaglia FL, Kermode AG. Low sensitivity of anti- aquaporin-4 antibody in multiple sclerosis, longitudinally extensive spinal cord lesions and neuromyelitis optica in Australians. Neurol Asia 2007;12:149-50. 60. Ravaglia S, Bastianello S, Franciotta D, Ceroni M, Pichiecchio A, Tavazzi E, et al. NMO-IgG-negative relapsing myelitis. Spinal Cord 2008 Dec 23. [E pub ahead of print]. 61. Mandler RN, Ahmed W, Dencoff JE. Devic's neuromyelitis optica: A prospective study of seven patients treated with prednisone and azathioprine. Neurology 1998;51:1219-20. 62. Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256-9. 63. Miyazawa I, Fujihara K, Itoyama Y. Eugene Devic (1858-1930). J Neurol 2002;249:351-2. 64. Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473-7. 65. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106-12. 66. Jacob A, Das K, Nicholas R, et al. Neuromyelitis optica (Devic's disease) in the United Kingdom: epidemiology, clinical, radiological and therapy profile in the first 42 patients. In: American Academy of Neurology 57th Annual Meeting; April 9-16, 2005, Miami Beach, Florida. Abstract PO5.133. 67. Wingerchuk DM, Hogancamp WF, O'Brien PC, et al. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107-14.
- 22. 68. Cree BA, Goodin DS, Hauser SL. Neuromyelitis optica. Semin Neurol 2002;22:105-22. 69. Pittock SJ, Lennon VA, Krecke K, et al. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390-6. 70. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450-61. 71. Compston A. 'The marvellous harmony of the nervous parts': the origins of multiple sclerosis. Clin Med 2004;4:346-54. 72. Metwally, MYM: Textbook of neuroimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 11.2a. April 20100 73. Case of the week ..Acute postinfectious transverse myelitis (Transverse myelitis spectrum of disorders) (Click to download in PDF format] 74. Online case record...Acute postinfectious transverse myelitis [Full text] 75. Case of the week..............Acute postinfectious transverse myelitis [Click to download in PDF format] 76. Thesis section Postinfectious monophasic demyelinating disorders of the CNS [Click to download in PDF format] 77. Topic of the month Transverse myelitis. [Click to download in PDF format] 78. Topic of the month Neuromyelitis optica. [Click to download in PDF format] 79. Case of the week Acute postinfectious transverse myelitis [Click to download in PDF format] 80. Case of the week Acute postinfectious transverse myelitis [Click to download in PDF format] 80. A quick guide to management Neuromyelitis optica [Click to download in PDF format] 82. Devic’s Disease (Neuromyelitis Optica [Full text]
